Distribution and innervation of lateral line organs in the channel catfish
Abstract
The lateral line system of the channel catfish is formed by mechanoreceptive neuromasts located within five pairs of cephalic and one pair of trunk canals, as well as superficial lines of neuromasts, termed accessory and/or pit lines. Five pairs of pit lines occur on the head, and three pairs of superficial lines occur on the trunk. In addition to these mechanoreceptors, which are found in most teleost fishes, catfish also possess a total of over 4000 electroreceptive ampullary organs scattered over the entire body. The lateral line receptors are innervated by five pairs of lateral line nerves whose rami are secondarily associated with facial and trigeminal fibers that innervate taste buds and the dermis of the skin, respectively. The neuromasts of the trunk canal and the ramules of the posterior lateral line nerve that innervate them seem to be organized in a segmental pattern. The same is true for the intervertebral ramules of the recurrent facial ramus, which innervate the external taste buds on the trunk. The fibers of the gustatory and lateral line systems may use the neural crest, the developing spinal nerves, or both, to establish this segmental pattern. In this context, it may not be surprising that there is an intimate relationship among each of the sensory systems in the trunk. J. Comp. Neurol. 421:570–592, 2000. © 2000 Wiley-Liss, Inc.
The lateral line system of many fishes and amphibians consists of mechanoreceptive neuromasts and electroreceptive ampullary organs (Hetherington and Wake, 1979; Fritzsch and Wahnschaffe, 1983; Northcutt, 1986, 1992a; Coombs et al., 1988). Neuromasts are composed of a centrally elongated strip of directionally sensitive hair cells surrounded by a peripheral zone of mantle and support cells, and they are stimulated by low frequency water movements parallel to the major axes of the receptors (Flock, 1965; Denton and Gray, 1989). An outgroup analysis (i.e., a step-wise examination of a given trait, beginning with the most closely related taxon and progressing to more distantly related taxa) of living and fossil fishes (Northcutt, 1989, Northcutt, 1997) indicates that the neuromasts of the earliest fishes were arrayed in lines and housed in a complex series of grooves extending over the head and onto the trunk. These fishes apparently possessed at least 11 lines of neuromasts on the head and up to three lines of neuromasts on the trunk. The subsequent phylogenetic history of neuromast lines is characterized by four trends: (1) the failure of grooves to form, resulting in superficial lines of neuromasts; (2) a reduction in one or more segments of a line of neuromasts; (3) the loss of an entire line of neuromasts; and (4) the origin of adjacent lines of neuromasts (accessory neuromast lines). Both reduction and complete loss of one or more neuromast lines has occurred in every group of extant fishes and amphibians. Interestingly, teleost fishes exhibit the most extensive reduction, loss, or both, of phylogenetically older neuromast lines but are also the only group of fishes to have evolved new accessory neuromast lines (Coombs et al., 1988; Puzdrowski, 1989).
Electroreceptive ampullary organs, the second class of lateral line receptors, occur widely among fishes (lampreys, cartilaginous fishes, sturgeons and paddlefishes, and lungfishes, Northcutt, 1986; New, 1997), as well as in two of the three orders of living amphibians (Fritzsch and Wahnschaffe, 1983; Wahnschaffe et al., 1985; Northcutt, 1992a). In all of these taxa, ampullary organs occur as epidermal invaginations in which a lumen, open to the skin surface, ends as a deep epithelial ampulla consisting of support and receptor cells. The apical surface of each receptor cell usually bears a single kinocilium (Jørgensen et al., 1972; Teeter et al., 1980; Northcutt and Brändle, 1995) and, frequently, modified microvilli (stereocilia). Several surveys (Fritzsch and Wahnschaffe, 1983; Wahnschaffe et al., 1985; Northcutt, 1986, Northcutt, 1992b; New, 1997) indicate that, primitively, ampullary organs are restricted to the head (except in lampreys and lungfishes) and exhibit their highest density on the snout and around the eyes. Their distribution is not random, as they are closely associated with the neuromast lines and arise from the same placodes as do the neuromasts (Northcutt and Brändle, 1995; Northcutt et al., 1995). Ampullary electroreceptors were apparently lost with the origin of neopterygian fishes, because they are not present in bowfins and gars, the living representatives of the oldest neopterygian radiation (McCormick, 1982; Bullock et al., 1983), nor do they occur in most living teleosts, the last and most successful group of neopterygians to evolve. In two groups of teleosts, some osteoglossomorph and ostariophysan fishes, however, electroreceptors seem to have independently re-evolved. Teleost electroreceptors differ extensively from those of nonteleost fishes and amphibians with regard to their histology, innervation, central projections, and physiology (Maler et al., 1973; Szabo, 1974; Roth and Tscharntke, 1976; Hetherington and Wake, 1979; Münz et al., 1984; Bullock and Heiligenberg, 1986).
The cranial nerves that innervate lateral line receptors also exhibit considerable phylogenetic variation. Although lateral line receptors were initially thought to be innervated by rami of the facial, glossopharyngeal, and vagal nerves (Herrick, 1899; Coghill, 1902; Norris, 1925), subsequent descriptive and experimental studies (Maler et al., 1973; Boord and Campbell, 1977; Puzdrowski, 1989; Song and Northcutt, 1991; Northcutt, 1992a; Northcutt and Bemis, 1993; Piotrowski and Northcutt, 1996) have revealed that they are instead innervated by up to six pairs of distinct lateral line nerves. An outgroup analysis of variation in the number of lateral line nerves and the pattern of electroreceptor innervation indicates that five of the six pairs innervate electroreceptors in the primitive condition (Northcutt, 1986, Northcutt, 1997). Not surprisingly, electroreceptors that re-evolved independently in various groups of teleost fishes are innervated by a variable number of lateral line nerves (Maler et al., 1973; Bell and Russell, 1978; Carr and Matsubara, 1981; Braford, 1986; Northcutt and Vischer, 1988; New and Singh, 1994). In addition to variation in their innervation of electroreceptors, lateral line nerves in teleosts vary in other ways: one or more lateral line nerves can be lost or secondarily fused with another lateral line nerve or closely associated branchiomeric nerve. However, unlike lateral line receptors, no new lateral line nerves have evolved.
Over the past 20 years, a wealth of information regarding the variation in lateral line receptors and their innervation has been generated, and researchers have also identified where in vertebrate phylogeny major changes have occurred. However, little progress has been made in determining how these phylogenetic changes occur. Ontogenetic studies will allow us to identify the embryonic source(s) of lateral line receptors and nerves and discern the mechanisms responsible for their change over time. Garstang (1922) was the first to discern the fundamental relationship between ontogeny and phylogeny when he realized that phylogeny is not a succession of adult forms. Rather, it is the result of successive changes over time in an ancestral life history. Thus, ontogeny does not recapitulate phylogeny; changes in ontogenies create phylogeny. Because the ontogeny of a common ancestor cannot be examined, the ontogenies of an ancestor's living descendants must be examined. An outgroup analysis of multiple ontogenies (Northcutt, 1992b) can determine which stages are primitive (i.e., were present in a common ancestor) and which are derived (i.e., have arisen subsequently). To understand the ontogenetic changes underlying interspecific variation in neuromast lines, the loss and re-evolution of electroreceptors, and the innervation of both types of lateral lines, it is necessary to compare the ontogenies of a species that develops both neuromasts and primitive electroreceptors, a species that develops neuromasts but not electroreceptors, and a teleost species that develops neuromasts and the newly evolved electroreceptors. Salamanders are the only group of vertebrates in which it is practical to study the development of primitive electroreceptors, and an extensive descriptive and experimental literature (Stone, 1922; Smith et al., 1988, Smith et al., 1990; Northcutt, 1992a; Northcutt and Brändle, 1995; Northcutt et al., 1995) exists. Although numerous neopterygian fishes are suitable for examining a species that develops neuromasts but no electroreceptors, channel catfishes (Ictalurus punctatus) are an ideal group in which to examine the development of the newly evolved electroreceptors. Channel catfish have the advantage that they are raised commercially so that embryos are available in large numbers, and its developmental stages have been described (Armstrong and Child, 1962).
Although Herrick recognized the importance of developmental studies, he also believed “that studies in development should be preceded by a thorough knowledge of the adult structures involved,” (Herrick, 1901, p. 179). Accordingly, Herrick undertook a detailed study of the cranial nerves and cutaneous sense organs of the black bullhead catfish, Ameirus melas. Although it is possible that there are no major neuroanatomical differences between Ameirus and Ictalurus, developmental studies of channel catfishes cannot be undertaken with confidence in the absence of a detailed description of their adult lateral line system. Furthermore, even if there are no major differences in the lateral line system of these two genera, new descriptive and experimental neuroanatomical techniques now exist that were not available to Herrick. For these reasons, a detailed study of the lateral line system of channel catfishes was undertaken as a necessary preamble to the study of the development of this system.
Abbreviations
-
- af
-
adipose fin
-
- agc
-
anterior ganglionic complex
-
- AN
-
accessory neuromast
-
- an
-
anterior naris
-
- anf
-
anal fin
-
- ao
-
ampullary organ
-
- AP
-
accessory pit canal and line
-
- ap
-
apical pore of ampullary organ
-
- ar
-
anterior ramule of the superficial ophthalmic ramus
-
- aro
-
anterior ramus of octaval nerve
-
- ATL
-
accessory trunk line
-
- AV
-
anteroventral lateral line nerve
-
- buc
-
buccal ramus of anterodorsal lateral line nerve
-
- c
-
corium of taste bud
-
- cc
-
corpus cerebelli
-
- cct
-
cerebellar crest
-
- cf
-
caudal fin
-
- ch
-
caudal ramule of hyoid ramus
-
- cp
-
trunk canal pore
-
- d
-
dorsal ramus of posterior lateral line nerve
-
- df
-
dorsal fin
-
- dgVII
-
dorsal subdivision of facial sensory ganglion
-
- DL
-
dorsal trunk line
-
- dmd
-
deep subdivision of the anteroventral lateral line nerve
-
- dr
-
dorsal ramule of lateral ramus of posterior lateral line nerve
-
- dra
-
dorsal ramule of dorsal somatic ramus
-
- drg
-
dorsal root ganglion of spinal nerve
-
- dsr
-
dorsal somatic ramus of spinal nerve
-
- dV
-
descending trigeminal tract
-
- ec
-
epithelial canal
-
- eg
-
eminentia granularis
-
- ell
-
electrosensory lateral line lobe
-
- EN
-
ethmoid neuromast
-
- fl
-
facial lobe
-
- fr
-
fin rays of caudal fin
-
- gAD
-
sensory ganglion of anterodorsal lateral line nerve
-
- gAV
-
sensory ganglion of the anteroventral lateral line nerve
-
- gM
-
sensory ganglion of middle lateral line nerve
-
- gO
-
sensory ganglion of otic lateral line nerve
-
- gP
-
sensory ganglion of posterior lateral line nerve
-
- gV
-
trigeminal sensory ganglion
-
- gVII
-
sensory ganglion of facial nerve
-
- gIX
-
sensory ganglion of glossopharyngeal nerve
-
- h
-
hyoid ramus of facial nerve
-
- hyo
-
hyomandibular trunk
-
- ib
-
inner buccal ramus of anterodorsal lateral line nerve
-
- il
-
inferior lobe
-
- im
-
inferomedial strand
-
- IO
-
infraorbital canal
-
- ivr
-
intervertebral ramule of facial recurrent ramus
-
- l
-
lateral ramus of posterior lateral line nerve
-
- lr
-
lateral ramule of a ventral somatic ramus
-
- lg
-
lateral sensory ganglia of vagal nerve
-
- lll
-
lateral line lobes
-
- m
-
middle lateral line nerve
-
- ma
-
mantle cells of lateral line organs
-
- mab
-
mandibular barbel
-
- man
-
mandibular ramus of trigeminal nerve
-
- MAP
-
mandibular pit line
-
- max
-
maxillary ramus of trigeminal nerve
-
- meb
-
mental barbel
-
- mg
-
medial sensory ganglion of vagal nerve
-
- mh
-
medial ramule of hyoid ramus
-
- mon
-
medial octavolateral nucleus
-
- MP
-
middle pit line
-
- mr
-
medial ramule of a ventral somatic ramus
-
- mxb
-
maxillary barbel
-
- myo
-
myomere
-
- mys
-
myoseptum
-
- mVII
-
facial motor nucleus
-
- nb
-
nasal barbel
-
- ne
-
neural elements of dorsal and ventral arches
-
- O
-
otic canal
-
- ob
-
outer buccal ramus of anterodorsal lateral line nerve
-
- oc
-
otic capsule
-
- on
-
optic nerve
-
- op
-
olfactory peduncle
-
- ope
-
opercular ramus of facial nerve
-
- or
-
otic lateral line nerve
-
- ot
-
optic tectum
-
- pal
-
palatine ramus of facial nerve
-
- pecf
-
pectoral fin
-
- pef
-
position of pelvic fin
-
- pelf
-
pelvic fin
-
- pf
-
position of pectoral fin
-
- pn
-
posterior naris
-
- pp
-
posterior palatine ramus of facial nerve
-
- pr
-
sensory root of the posotic lateral line nerves
-
- prf
-
profundal nerve
-
- PRM
-
preoperculomandibular canal
-
- pro
-
posterior ramus of the octaval nerve
-
- QJ
-
quadratojugal pit line
-
- rh
-
rostral ramule of hyoid ramus
-
- ri
-
rib
-
- rp
-
sensory root of the posterior lateral line nerve
-
- rr
-
recurrent ramus of the facial nerve
-
- s
-
support cells of lateral line organs
-
- sAD
-
sensory root of anterodorsal lateral line nerve
-
- sc
-
sensory cells of lateral line organs
-
- se
-
stratified squamous epithelium
-
- sf
-
sensory fibers of facial nerve
-
- sl
-
superolateral strand
-
- sm
-
sensory macula of neuromast
-
- smd
-
superficial subdivision of the combined anteroventral lateral line and facial nerves
-
- sn
-
first spinal nerve
-
- SO
-
supraorbital canal
-
- so
-
superficial ophthalmic ramus of anterodorsal lateral line nerve
-
- sp
-
sensory papilla
-
- spc
-
spinal cord
-
- sr
-
sensory roots of preotic lateral line nerves
-
- st
-
sensory fibers of trigeminal nerve
-
- T
-
temporal canal
-
- tb
-
taste bud
-
- TC
-
trunk canal
-
- td
-
dorsal ramules of terminal dorsal and ventral somatic rami
-
- tds
-
terminal dorsal somatic ramus
-
- tel
-
telencephalon
-
- tf
-
trigeminal fibers that anastomose with the anterior ramule
-
- tlc
-
trunk lateral line canal
-
- tv
-
ventral ramules of terminal dorsal and ventral somatic rami
-
- tvs
-
terminal ventral somatic ramus
-
- v
-
ventral ramus of posterior lateral line nerve
-
- vgVII
-
ventral subdivision of facial sensory ganglion
-
- vir
-
visceral rami of vagal nerve
-
- VL
-
ventral trunk line
-
- vl
-
vagal lobe
-
- VLN
-
vertical line of neuromasts at base of the caudal fin
-
- vr
-
ventral ramules of lateral and ventral rami of posterior lateral line nerve
-
- vra
-
ventral ramule of a dorsal somatic ramus
-
- vsr
-
ventral somatic ramus of spinal nerve
-
- vt
-
vertebra
-
- VIII
-
octaval nerve
-
- IX
-
glossopharyngeal nerve
-
- X
-
vagal nerve
-
- 1b
-
first branchial ramus of vagal nerve
-
- 2b
-
second branchial ramus of vagal nerve
-
- 3b
-
third branchial ramus of vagal nerve
MATERIALS AND METHODS
All experiments and observations involved juvenile and adult channel catfish, Ictalurus punctatus, obtained from Carolina Biological Supply Company in Burlington, North Carolina. All procedures were approved by the UCSD Animal Care and Use Committee and conform to NIH guidelines.
To identify the different morphological classes of lateral line receptors and map their distribution, juveniles (2.5 to 11.5 cm in total length) were anesthetized in a dilute solution of tricaine methane-sulfonate (MS222, Sigma Chemical Co., St. Louis, MO) and fixed by immersion in 4% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). After fixation for at least 1 week, the animals were rinsed in phosphate buffer, and flat-mounts of the head and trunk skin (Lannoo, 1985; Northcutt, 1992a) were prepared according to the following procedure. Mid-dorsal and mid-ventral incisions were made along the entire length of the animal, and the skin was carefully separated from the connective tissue and muscle by using number 5 Dumont forceps (Roboz Surgical Instrument Co., Inc., Washington, DC). To achieve this separation, one tine of the forceps was inserted into the dorsal or ventral midline incision, parallel to the inner surface of the skin, then slowly oscillated and advanced under the skin, separating it from the underlying tissues. The skin was placed in 3% hydrogen peroxide for approximately 12 hours to bleach pigment cells that would otherwise obscure details of the lateral line receptors. The skin samples were then washed in 0.1 M phosphate buffer and stained in l% methylene green (Sigma). The samples were stained free-floating and then dehydrated in a graded series of ethanols and cover-slipped on slides. Maps of the distribution of the receptors (Figs. 1, 3) were prepared by tracing the projected images of the skin samples with the aid of a camera lucida (Olympus Optical Co., Ltd., Tokyo, Japan).
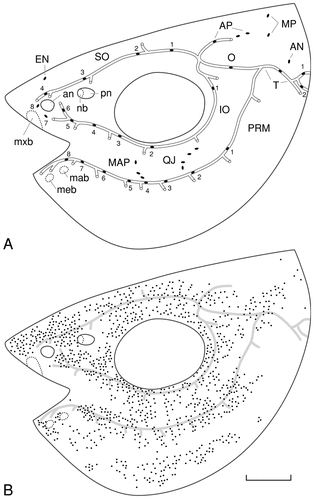
Camera lucida drawings showing the position of lateral line receptors on the head of a juvenile channel catfish (6.8 cm total length). A: Each neuromast, whether within a canal or on the surface of the skin, is indicated by a solid oval. The short tubes branching from the main canals are pores where the canals open to the surface. Generally only a single neuromast is located within a canal between two pore openings. B: The positions of individual electroreceptive ampullary organs (black dots) are mapped in relation to the more deeply located lateral line canals indicated in gray. For abbreviations, see list. Scale bar = 2 mm in B (applies to A,B).

Camera lucida drawings of the rostral (A) and caudal (B) trunk of the same juvenile channel catfish illustrated in Figure 1 showing the position of individual canal and superficial neuromasts. Note that the major axes of the neuromasts of the short dorsal line (DL), as well as those of the trunk canal (TC) and ventral line (VL), are parallel to the longitudinal axis of the body and are sensitive to relative movement of water only in the longitudinal axis, whereas the major axes of the neuromasts of the short accessory lines (ATL) above the trunk canal are perpendicular to all the other neuromasts and are sensitive to relative movement of water in the dorsal-ventral axis. For abbreviations, see list. Scale bar = 4 mm in B (applies to A,B).
Camera lucida drawings of the rostral (A) and caudal (B) trunk of the same catfish, indicating the distribution of individual electroreceptive ampullary organs. The position of the more deeply located trunk canal is indicated in gray. For abbreviations, see list. Scale bar = 4 mm in B (applies to A,B).
The skin of juveniles up to 12 cm in total length remains sufficiently thin to visualize easily the details of the larger electroreceptors and taste buds but not the neuromasts. To make more detailed histologic observations of the lateral line receptors, two 55-day posthatching larvae (3 to 4 cm in total length) were fixed by immersion in 4% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). After fixation, the heads were washed in distilled water for 12 hours and then dehydrated in a graded series of ethanols. When the specimens reached 95% ethanol, they were infiltrated and embedded in glycol methacrylate (Leica Instruments, Heidelberg, Germany), cut into serial sections (4 to 5 μm), and stained with 1% cresyl violet (Sigma).
The course of the peripheral rami and the position of the ganglia of the cranial nerves that innervate the lateral line receptors were visualized by several different approaches: gross dissection, staining of the nerves in whole cleared animals by Sudan Black (Filipski and Wilson, 1984), tracing the individual rami in serial transverse sections of the whole head of a juvenile (7.8 cm total length) stained by the Bodian-reduced silver procedure (Senn, 1968), or similar tracing in glycol methacrylate-embedded sections of the 55-day posthatching larva and/or serial transverse sections stained with cresyl violet of the pre- and postotic cranial ganglia of an adult catfish.
Finally, the rami of many of the cranial nerves suspected to contain fibers innervating lateral line receptors were labeled with horseradish peroxidase (HRP, Sigma, type VI) to determine whether these rami contain fibers that terminate centrally in the electrosensory lateral line lobes (electroreceptors) or the medial octavolateralis nucleus (mechanoreceptors), the known primary targets of the afferent lateral line fibers (Finger and Tong, 1984; New and Singh, 1994).
When HRP was used to label various rami, the animals were anesthetized in a dilute solution of MS222, a flap of skin was reflected, and the connective tissue or muscle was retracted to expose a nerve ramus. The ramus was transected, and a small piece of Gelfoam (Upjohn Co.), saturated with a solution of 40% HRP dissolved in 1% lysophosphatidylcholine (Sigma) in distilled water, was applied to the proximal stump of the ramus. The incision was closed and sutured. Care was taken that the HRP-soaked Gelfoam did not come into contact with other rami. After survival times of 4–14 days at 26–28°C, the animals were reanesthetized in a dilute solution of MS222 and perfused transcardially with cold 0.1 M phosphate buffer (pH 7.4), followed by 4% glutaraldehyde dissolved in phosphate buffer. The brains and nerves were removed, postfixed for 1 to 3 hours in the same fixative, then embedded, cut into 35-μm transverse sections, and reacted (Adams, 1981) as free floating sections. The sections were then mounted on chrome-alum coated slides and counterstained with 1% neutral red (Sigma).
The following nerves, trunks, or rami were labeled with HRP to establish the sensory components carried in them: superficial ophthalmic ramus (N3), buccal and maxillary rami (N6), mandibular ramus (N5), profundal nerve (N3), mandibular ramus (N5), hyomandibular trunk (N4), hyoid ramus (N2), deep mandibular ramus (N2), superficial mandibular ramus (N2), posterior lateral line nerve (N6), recurrent facial ramus (N5), and visceral rami of vagal nerve (N2). We were not able to label the otic or middle lateral line nerves, nor did we try to label the dorsal and ventral rami of the posterior lateral line nerve.
Computer graphics applications were used in the preparation of photographic material. Operations included adjustment of brightness, contrast, and color tone, application of labels, and masking of background regions by using Adobe Photoshop 5.0.2 (Adobe Systems, San Jose, CA).
RESULTS
The lateral line system of channel catfishes consists of two morphological classes of receptors: canal and superficial mechanoreceptive neuromasts and electroreceptive ampullary organs. The morphology and distribution of these receptors are described first (Figs. 1-4), followed by a description of the rami and ganglia of the cranial nerves that innervate these receptors (Figs. 5-13).
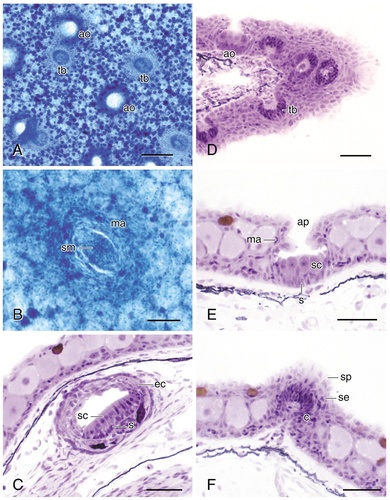
Photomicrographs of flat-mounted skin (A,B) and 5-μm plastic-embedded sections of ampullary organs (A,D,E), canal (C) and pit line (B) neuromasts, and taste buds (A,D,F). The ampullary organs and taste buds illustrated in D are located at the base of a maxillary barbel, which was sectioned in the transverse plane, as were the organs in C–F. For abbreviations, see list. Scale bar = 100 μm in A; 50 μm in B; 25 μm in C–F.
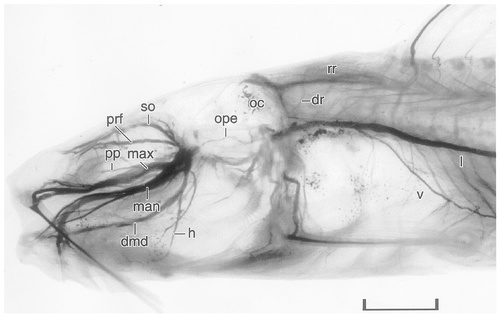
Photomicrograph of the cleared head of a juvenile channel catfish stained with Sudan Black to reveal the peripheral course of the cranial nerves. The palatine ramus of the facial nerve is hidden in this photomicrograph by the maxillary ramus of the trigeminal nerve. For abbreviations, see list. Scale bar = 4 mm.
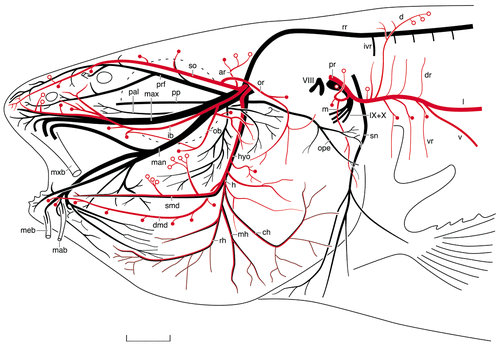
Camera lucida drawing of a Sudan Black preparation of another juvenile channel catfish, illustrating details of the cranial nerves. The rami of the various lateral line nerves are indicated in red, and the canal and superficial neuromasts are indicated by solid red dots and open circles, respectively. For abbreviations, see list. Scale bar = 3 mm.
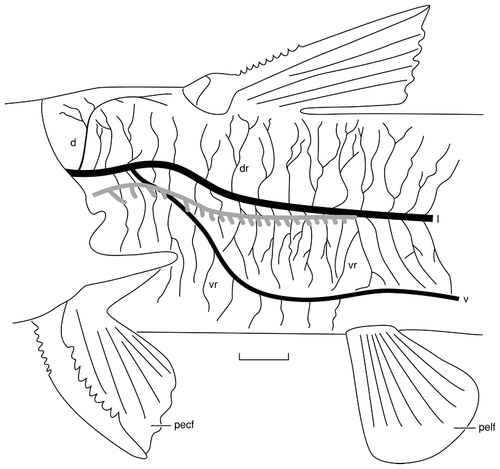
Camera lucida drawing of the trunk of a juvenile channel catfish stained with Sudan Black to reveal the regions innervated by the three rami of the posterior lateral line nerve. The trunk canal, indicated in gray, is located ventral to the lateral ramus rostrally but comes to lie immediately lateral to the ramus more caudally. The canal is not drawn here because it would obscure details of the lateral ramus. The dorsal ramus innervates only a few ampullary organs and superficial neuromasts rostrally adjacent to the dorsal fin, whereas the lateral ramus innervates all the remaining lateral line organs located dorsal to the ventral ramus. The ventral ramus innervates the neuromasts of the ventral trunk line and all ampullary organs located in the skin of the belly. For abbreviations, see list. Scale bar = 5 mm.
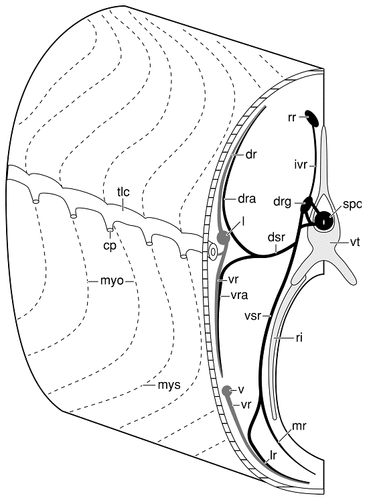
Schematic drawing of the left side of the trunk (rostral is to the left on the figure) of a catfish, illustrating the intimate relationships of the recurrent facial ramus, a spinal nerve, and the lateral and ventral rami of the posterior lateral line nerve. Taste buds on the trunk are innervated by sensory fibers that leave the facial recurrent ramus to enter the dorsal root ganglia of spinal nerves by means of intervertebral ramules. The facial fibers then enter dorsal and ventral somatic rami of spinal nerves where they continue to the skin in consort with the somatic sensory fibers of these nerves. There is a similar relationship between the spinal nerves and the fibers of the lateral and ventral rami of the posterior lateral line nerve. The ramules of the lateral and ventral rami anastomose with some of the elements of the spinal nerves, so that somatic sensory, gustatory, and lateral line fibers ramify beneath the skin in the same fiber fascicles. For abbreviations, see list.
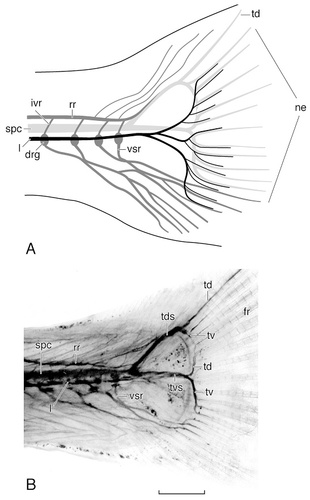
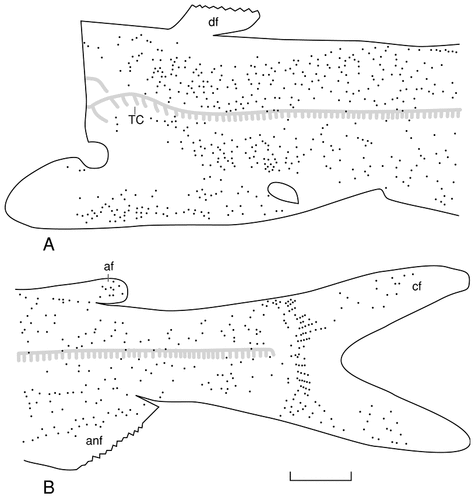
Camera lucida drawing (A) and photomicrograph (B) of the cleared caudal fin of a juvenile channel catfish stained with Sudan Black, illustrating the terminal arborization of the lateral ramus of the posterior lateral line nerve. The lateral line fibers that enter and terminate within the caudal fin do so in consort with fibers of the caudal spinal nerves and facial recurrent ramus. For abbreviations, see list. Scale bar = 200 μm in B (applies to A,B).
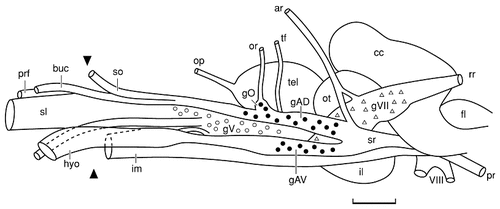
Camera lucida drawing of the lateral surface of the brain and anterior ganglionic complex of an adult channel catfish. The anterior ganglionic complex, a derived feature of teleost fishes, is formed by the partial fusion of the ganglia of the preotic lateral line nerves (solid circles), the ganglion of the trigeminal nerve (open circles), and the ganglion of the facial nerve (open triangles). The extent of each of the ganglia is based on analysis of serial sections of a second individual of approximately the same length. Only the ganglia of the preotic lateral line nerves can be seen in entirety because of their lateral, superficial position within the complex. The total length of the more medially located trigeminal ganglion can be seen because of the separation of the anterodorsal and anteroventral lateral line nerves. The dorsal and ventral borders of the trigeminal ganglion coincide with the ventral half and dorsal half of the trunks of the anterodorsal and anteroventral lateral line nerves, respectively. The facial ganglion is the most medial and the largest cell group in the complex. The caudal segment of the facial ganglion extends dorsally to the entering roots of the preoptic lateral line nerves, and it extends rostrally and ventrally to occupy the ventromedial half of the entire complex. The arrowheads at the left of the figure indicate the position where various rami of these nerves exit the neurocranium. For abbreviations, see list. Scale bar = 2 mm.
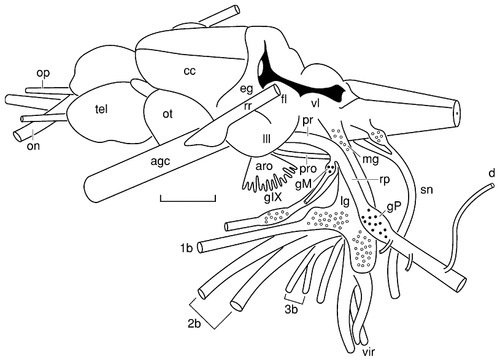
Camera lucida drawing of the cranial nerves and brain of an adult catfish. The perspective is dorsolateral with respect to the anteroposterior axis of the brain. The position and extent of the sensory ganglia of the postotic branchiomeric (open circles) and lateral line (solid circles) nerves are based on analysis of serial sections of a second individual of approximately the same length. For abbreviations, see list. Scale bar = 3 mm.
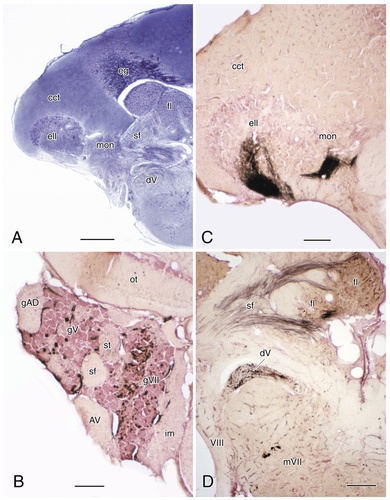
A: Photomicrograph of a Bodian-stained transverse section through the rostral medulla and cerebellum of a channel catfish. B: Transverse section through the anterior ganglionic complex with anterogradely labeled sensory neurons of the facial and trigeminal ganglia. C: Transverse section through the rostral medulla and cerebellum with anterogradely labeled sensory fibers of the posterior lateral line nerve entering the electrosensory lateral line lobe and medial octavolateral nucleus: C: transverse section through the medulla and cerebellum at a level comparable to that in A, with anterogradely labeled sensory fibers of the facial and trigeminal nerves, as well as retrogradely labeled facial motor neurons, after application of horseradish peroxidase to the superolateral strand (D). In each case, dorsal and lateral surfaces are to the top and left of the photomicrographs, respectively. For abbreviations, see list. Scale bar = 500 μm in A; 200 μm in B–D.
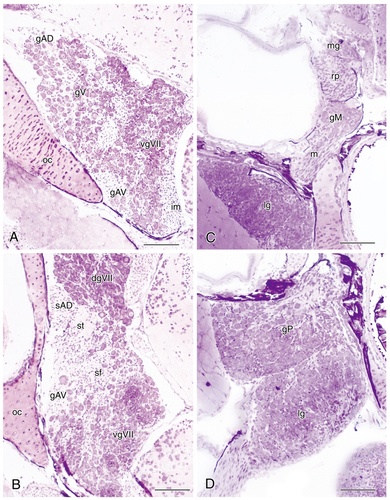
Photomicrographs of 5-μm-thick plastic-embedded transverse sections through sensory ganglia of various cranial nerves in the channel catfish rostral A and caudal B levels through the anterior ganglionic complex showing the positions of the anterodorsal and anteroventral lateral line ganglia relative to the ganglia of the facial and trigeminal nerves. C: Section through the ganglion of the middle lateral line nerve illustrating its position relative to the root of the posterior lateral line nerve and the medial and lateral vagal ganglia. D: Section through the sensory ganglia of the posterior lateral line and vagal nerves at their closest apposition. Dorsal and lateral are to the top and left of each panel, respectively. For abbreviations, see list. Scale bar = 100 μm in A–D.
Morphology of receptors
Both canal and superficial neuromasts (Fig. 4B,C) are larger than ampullary organs (Fig. 4A,D,E), and both types of neuromasts are elliptically shaped with an elongated central zone of hair cells that forms a sensory macula (Fig. 4B,C). The hair cells of the macula (Fig. 4C) are scattered among support cells, and the outer borders of both types of neuromasts are surrounded by very small mantle cells (Fig. 4B). Measurements of the major axes of superficial neuromasts, termed large pit organs by Herrick (1901), on the head and trunk in the flat-mounted skin of a single 11.5-cm-long individual resulted in means of 149 μm ± SE 7.4 (N11) and 134 μm ± SE 4.5 (N23), respectively. Thus, cephalic superficial neuromasts are slightly larger than trunk superficial neuromasts, as are the major axes of their sensory macula: x = 79 μm ± SE 4.4 (N11) and x = 68 μm ± SE 2.2 (N23), respectively. Although it is impossible to measure canal neuromasts in flat-mounts of the skin, measurements of the major axis of cephalic canal neuromasts from serial sections of a smaller individual (7.8 cm total length) indicate that canal neuromasts are larger (x = 205 μm ± SE 13.0, N10) than either cephalic or trunk superficial neuromasts.
The smallest class of lateral line organs are the ampullary organs on the head and trunk (Fig. 4A,D,E), termed small pit organs by Herrick (1901), with a mean diameter of 100 μm ± SE 2.7 (N20) and 110 μm ± SE 3.6 (N20), respectively. Ampullary organs consist of a short, invaginated epithelial tube, open to the skin surface by an apical pore (Fig. 4D,E) that ends as a slightly swollen sensory bulb within the epidermis. The epithelial tube is ensheathed in small mantle cells (Fig. 4E), comparable to the mantle cells of neuromasts, and the sensory bulb consists of sensory and support cells (Fig. 4E).
In flat-mounts of the skin (Fig. 4A), ampullary organs might be confused with taste buds (Fig. 4A,D,F) which also occur in the epidermis of the head and trunk. Taste buds, termed terminal or end buds by Wright (1884a) and Herrick (1901), are slightly smaller than ampullary organs; however, when measured in the same individual (the mean diameter of cephalic taste buds was 86 μm SE 4.0, N20 and the mean diameter of trunk taste buds was 79 μm SE 4.1, N19). Ampullary organs can also be distinguished from taste buds in flat-mounts because ampullary organs possess apical pores (Fig. 4A,E), whereas taste buds exhibit sensory papillae (Fig. 4F) that are elevated above the surface of the epidermis. In transverse sections of the skin (Fig. 4D,E,F), the histological differences between ampullary organs (Fig. 4E) and taste buds (Fig. 4F) are even more pronounced. Taste buds are solid cellular organs composed of an inner corium and an outer layer of stratified squamous epithelium, and the receptor cells of taste buds are located superficially in a sensory papilla rather than at the bottom of a pit as is the case for ampullary organs.
Distribution of lateral line organs
Cephalic and trunk neuromasts (Figs. 1A, 2) may occur in canals or as superficial lines. On both the head and trunk, only a single neuromast occurs between short canal pores that open to the surface. Canal neuromasts on the head occupy five main canals (Fig. 1A). Four neuromasts occur in a supraorbital canal (SO) located above the orbit, and six neuromasts occur in an infraorbital canal (IO) below the orbit. These canals join caudal to the orbit, forming an otic canal (O) that houses a single neuromast. Slightly more caudal, the otic canal is joined by the more ventrally located preoperculomandibular canal (PRM), which houses eight neuromasts and is the only canal located on the lower jaw and cheek region. A single canal, the temporal canal (T), which also houses a single neuromast, continues caudally after the fusion of the otic and preoperculomandibular canals. The temporal canal in turn is continuous with the trunk canal (TC, Fig. 2), which houses approximately 100 neuromasts, with a single neuromast occurring per body segment. In addition to the five main cephalic canals, there is a short canal that encloses one of the three neuromasts of the anterior pit line (AP, Fig. 1A). Pit lines usually occur as superficial lines of neuromasts that are believed to represent phylogenetically older lines that primitively were enclosed in canals (Northcutt, 1989). Among ray-finned fishes, catfishes are unusual in that part of their anterior pit line forms a canal.
The remaining neuromasts on the head occur as superficial neuromasts. Rostrally, a single neuromast is located medial to the supraorbital line (EN, Fig. 1A), which seem to be homologous to the ethmoid line of other teleosts and two superficial neuromasts located ventral to the anterior naris, which seem to be the most rostral neuromasts of the infraorbital line based on their innervation (see next section). Two additional short lines of neuromasts occur on the lower jaw between the infraorbital and preoperculomandibular canals. On the basis of their topography, it is possible that these two lines are homologous to the mandibular and quadratojugal (QJ) pit lines (Fig. 1A). There is an additional pit line of two neuromasts dorsally, which is homologous to the middle pit line (MP) of other fishes based on its position and innervation (see next section). However, channel catfishes seem to have lost posterior and gular pit lines, as well as a supratemporal line.
The superficial neuromasts of the trunk (Fig. 2) are organized into four groups. Three neuromasts located rostral to the dorsal fin form an unusually short dorsal trunk line (DL, Fig. 2A). Associated with the trunk canal, there are vertically oriented accessory neuromast lines (ATL, Fig. 2A,B), which occur at intervals of six to eight body segments, and horizontally oriented neuromasts, which form a ventral trunk line (VL, Fig. 2A,B). The spacing of the neuromasts of the ventral trunk line is highly variable. A single neuromast may occur at one, two, or even three body segment intervals. Finally, there is a vertical line of nine horizontally oriented neuromasts at the base of the caudal fin (VLN, Fig. 2B).
Approximately 1,000 ampullary organs occur on each side of the head in juvenile channel catfish (Fig. 1B). The majority of these receptors are located adjacent to the neuromast lines, with the exception of a field of ampullary organs located on the hypobranchial surface of the lower jaw. Slightly fewer ampullary organs, approximately 800, occur on each side of the trunk (Fig. 3). Except for a patch of skin immediately caudal to the pectoral fin (Fig. 3A) that is devoid of ampullary organs, these organs are almost uniformly distributed on the trunk, with approximately equal numbers occurring dorsal and ventral to the trunk canal. Unlike neuromasts, ampullary organs occur on the adipose and anal fins and on the distal surfaces of the caudal fin (Fig. 3B). It has not been possible to determine the exact number of ampullary organs in adults, because the skin is too thick to treat as a flat-mount, but it is probable that ampullary organs continue to increase in number.
Innervation of lateral line organs
When intact heads and trunks stained with Sudan black (Fig. 5) are compared with gross dissections of the brain and sensory ganglia of the cranial nerves (Figs. 10, 11), it is possible to reconstruct the peripheral course of the nerves that innervate the lateral line organs (Figs. 6-9). Details of the sensory ganglia and peripheral rami of these nerves were further examined in sets of serial histologic sections through the intact head and trunk. As a final check on the accuracy of these reconstructions, many of the rami believed to contain fibers innervating lateral line organs were labeled with HRP. The sensory modality(ies) of each ramus that was experimentally examined was determined based on the following considerations: (1) the position of labeled neurons within the sensory ganglia of the cranial nerves (see Fig. 12B for an example); (2) whether or not the sensory root of a given cranial nerve contained labeled fibers; (3) whether or not the various medullary sensory tracts contained labeled fibers; and (4) the termination of the labeled fibers within the medulla. The axons of sensory ganglionic cells that innervate electroreceptive ampullary organs terminate in the electrosensory lateral line lobe, whereas the axons of sensory ganglionic cells that innervate mechanoreceptive neuromasts terminate in the medial octavolateral nucleus (Fig. 12A,C). It was also possible to verify experimentally that a given ramus contained somatic sensory fibers and/or special visceral sensory fibers of the gustatory system based on the presence of labeled fibers entering the descending trigeminal tract and the facial lobe, respectively (Fig. 12D). In theory, distinguishing trigeminal sensory fibers from facial sensory fibers in catfish could have been confounded by the termination of trigeminal fibers in the facial lobe (Kiyohara et al., 1986, Kiyohara et al., 1999). However, this did not prove to be the case because trigeminal fibers that terminate in the facial lobe initially course within the descending trigeminal tract, rather than in the facial sensory tract, before leaving the descending trigeminal tract to pass dorsally into the facial lobe. Therefore, it is possible to distinguish facial sensory fibers from trigeminal sensory fibers by the location of labeled neurons within the anterior ganglionic complex, by the location of labeled fibers within the primary sensory tracts, and by the way in which these fibers enter the facial lobe.
Based on these lines of evidence, as well as an outgroup analysis of other fishes and amphibians (Song and Northcutt, 1991; Northcutt, 1992a; Northcutt and Bemis, 1993; Piotrowski and Northcutt, 1996), we conclude that there is evidence that lateral line receptors in channel catfish are innervated by nine rami (Figs. 6, 7) that form five lateral line nerves: anterodorsal, anteroventral, otic, middle, and posterior lateral line nerves.
Anterodorsal lateral line nerve.
The ganglion of the anterodorsal lateral line nerve constitutes the most dorsolateral group of sensory neurons within the anterior ganglionic complex (Figs. 10, 12B, 13A). The ganglionic cells of this lateral line nerve (Fig. 13A) can be distinguished easily from the other neurons in the complex by their large size. Measurements of a random sample of anterodorsal lateral line ganglionic cells in a single 39-cm-long individual reveal a mean diameter of 18.7 μm ± SE 0.32 (N50). Not only are these sensory neurons among the largest ganglionic cells in the complex, they are scattered among fibers of the nerve trunk, which forms a separate, distinct bundle as it passes along the lateral surface of the ganglionic complex until its root joins that of the anteroventral lateral line nerve (Fig. 10). At this point, the combined roots enter the medulla and turn dorsally to terminate within the electroreceptive lateral line lobe, the medial octavolateral nucleus, and the eminentia granularis (Fig. 12A,C).
Superficial ophthalmic and buccal rami (Figs. 5, 6) arise from the rostral pole of the ganglion of the anterodorsal lateral line nerve (Fig. 10). As the superficial ophthalmic ramus passes rostrally, it exits the neurocranium by means of a separate foramen and continues rostrally above the orbit (Figs. 5, 6). Almost immediately, the superficial ophthalmic ramus gives rise to a ramule that innervates the first neuromast in the supraorbital canal (Figs. 1A, 6), and it also divides into several smaller fascicles that ramify in the skin. The profundal nerve also contributes fibers to the first ramule of the superficial ophthalmic ramus as this ramule emerges from the ramus (Fig. 6). Because the profundal nerve is joined by a sizable component of facial sensory fibers before its exiting the neurocranium, it is likely that this nerve consists of somatic and visceral sensory fibers. Both of these fiber classes probably also join the first ramule of the superficial ophthalmic ramus. If so, fibers of the first ramule innervate the skin and taste buds, as well as the first supraorbital neuromast and the ampullary organs closely associated with this segment of the supraorbital canal. As the superficial ophthalmic ramus passes dorsal to the eye, it issues a second ramule, which innervates the second supraorbital neuromast (Fig. 1A). This ramule also divides into several smaller fascicles that ramify in the skin. As the superficial ophthalmic ramus continues rostrally beyond the orbit to innervate the remaining two supraorbital neuromasts and the single ethmoid neuromast (Fig. 1A), it will anastomose with profundal rami at least two more times. Thus, it is likely that each superficial ophthalmic ramule also innervates skin and taste buds. Not surprisingly, application of HRP to the stump of the superficial ophthalmic ramus after transection at a midorbital level retrogradely labels cells in the anterodorsal lateral line ganglion, as well as cells in the facial and trigeminal ganglia whose labeled fibers can be traced into the facial lobe and descending trigeminal tract, respectively.
One additional ramule that arises from the dorsocaudal surface of the anterodorsal lateral line ganglion (Figs. 6, 10) is interpreted as an anterior ramule of the superficial ophthalmic ramus. In most bony fishes, this ramule arises from the proximal end of the superficial ophthalmic ramus rather than the position noted in catfish. However, in the latter, it is interpreted as the anterior ramule because it can be seen to innervate a single neuromast within a canal that arises from the caudal end of the supraorbital canal, as well as two superficial neuromasts (Figs. 1A, 6). In other fishes, this line of neuromasts is termed the anterior pit line and is always innervated by an anterior ramule of the superficial ophthalmic ramus. The anterior ramule is also joined by a small bundle of fibers that arises from the dorsal surface of the facial ganglion and, a little more distally, by an additional bundle of fibers that arises from the dorsal surface of the trigeminal ganglion (Fig. 10). Thus, the anterior ramule, like the other ramules of the superficial ophthalmic ramus, carries somatic and visceral sensory fibers that presumably innervate the skin and taste buds in the vicinity of the anterior pit line.
The buccal ramus of the anterodorsal lateral line nerve (Figs. 6, 10) also arises from the rostral pole of the anterodorsal lateral line ganglion. Unlike the superficial ophthalmic ramus, which courses dorsally to exit the neurocranium high on the wall of the orbit, the buccal ramus turns ventrally and exits the neurocranium as the most dorsolateral component of the superolateral strand (Fig. 10). In catfishes, most sensory and motor fibers initially emerge from the anterior ganglionic complex in one of three major bundles: the inferomedial strand, the superolateral strand, or the hyomandibular trunk (Fig. 10). Both the inferomedial and superolateral strands are composed of facial and trigeminal fibers. As the strands exit the neurocranium, some fibers are exchanged, but the inferomedial strand seems to be primarily composed of fibers that form the maxillary ramus of the trigeminal nerve and the palatine ramus of the facial nerve, whereas the superolateral strand is primarily composed of fibers that form the buccal ramus of the anterodorsal lateral line nerve and the mandibular ramus of the trigeminal nerve. In each case, the buccal, maxillary, and mandibular rami are composed, in part, of facial fibers that will innervate external taste buds. The hyomandibular trunk, the third major bundle of fibers to leave the anterior ganglionic complex, is formed by fibers of the anteroventral lateral line nerve, trigeminal somatic sensory fibers, and facial gustatory fibers.
The facial fibers that constitute part of the buccal ramus of the anterodorsal lateral line nerve arise from the dorsolateral surface of the superolateral strand and initially cap the buccal ramus (possibly a part of the accessory maxillary nerve of Herrick, 1901). The buccal ramus also seems to be joined by trigeminal fibers that arise from the ventrolateral surface of the superolateral strand and merge with the ventral surface of the buccal ramus. Unfortunately, all of the experimental cases that might have corroborated these observations included both the buccal and maxillary rami. In each of these cases, retrogradely labeled cells were observed in the facial and trigeminal ganglia, as well as in the anterodorsal lateral line ganglion, but labeled cells would have been expected in the facial and trigeminal ganglia if only the maxillary ramus were labeled.
The buccal ramus exits the neurocranium adjacent to the dorsolateral surface of the superolateral strand (Fig. 11). At this point, fibers are still being exchanged between the superolateral and inferomedial strands, but this exchange stops abruptly, and the maxillary and mandibular rami of the trigeminal nerve emerge as distinctly separate fiber bundles. As the maxillary ramus continues rostrally and ventrally, the buccal ramus remains adjacent to the dorsolateral surface of the maxillary ramus, then continues rostrally and divides into outer (ob, Fig. 6) and inner (ib, Fig. 6) branches, historically termed the outer and inner buccal rami (Herrick, 1901). The outer ramus turns laterally to pass under the m. levator arcus palatini and issues its first ramule, which innervates the first neuromast within the infraorbital canal (Figs. 1A, 6). Several additional branches issue from the first ramule (Fig. 6) and ramify within the skin innervating ampullary organs and taste buds in the vicinity of the caudal infraorbital canal. The remaining fibers of the outer buccal ramus continue rostrally to innervate the second neuromast of the infraorbital canal (Figs. 1A, 6). As these fibers course rostrally, numerous small twigs enter the skin so that most of the skin and associated sensory receptors located between the posterior half of the infraorbital and preoperculomandibular canals are innervated by the outer buccal ramus. The most rostrally coursing fibers of the outer buccal ramus anastomose with one or two branches of the inner buccal ramus to innervate the third neuromast of the infraorbital canal (Figs. 1A, 6).
Whereas the outer buccal ramus initially courses laterally and begins to innervate neuromasts of the caudal infraorbital canal and ampullary organs adjacent to the canal (Fig. 1B), the inner buccal ramus continues rostrally along the floor of the orbit, sandwiched between the maxillary and mandibular trigeminal rami. A brief anastomosis occurs between the inner buccal and maxillary rami at a midorbital level, after which the inner buccal ramus divides into lateral and medial ramules. As the lateral ramule courses rostrally, it issues one or two small fiber bundles, as already noted, which anastomose with the outer buccal ramus to innervate the third neuromast within the infraorbital canal.
Both ramules of the inner buccal ramus continue rostrally and laterally toward the rostral orbital wall. At this level, the lateral ramule issues numerous small branches that ramify within the skin, and then a larger branch that innervates the fourth neuromast within the infraorbital canal. The smaller branches of the lateral ramule almost certainly innervate the ampullary organs as well as the taste buds adjacent to the rostral orbit, because the only other nerve within this region, the maxillary ramus, remains encapsulated in connective tissue and does not issue any ramules.
The lateral ramule of the inner buccal ramus continues rostrally to innervate the fifth and sixth neuromasts within the infraorbital canal (Figs. 1A, 6). The lateral ramule then continues lateral to the olfactory capsule where it again forms a brief anastomosis with the maxillary trigeminal ramus before repeatedly dividing into smaller branches that ramify within the skin on the lateral surface of the snout. The medial ramule of the inner buccal ramus has paralleled the lateral ramule but now passes ventral to the olfactory capsule and does not seem to innervate any structures until it turns dorsally around the rostral pole of the olfactory capsule. At this point, it divides into two major bundles, each of which innervates one of the two superficial neuromasts (numbers 7 and 8) of the infraorbital line (Figs. 1A, 6). Both bundles also give rise to numerous small fascicles that ramify within the dorsolateral surface of the snout just prior to innervating the superficial neuromasts.
Anteroventral lateral line nerve.
The ganglion of the anteroventral lateral line nerve is located ventral to the ganglion of the anterodorsal lateral line nerve and lateral to the ventral subdivision of the facial sensory ganglion (Figs. 10, 13A,B). The cells of the anteroventral lateral line ganglion have essentially the same diameter (x = 19.5 μm SE ± 0.27, N50), as the cells of the anterodorsal lateral line ganglion in the 39-cm-long catfish. In addition to being slightly larger, cells of both the lateral line ganglia have a paler cytoplasm than cells of the adjacent ganglia within the anterior ganglionic complex. The sensory root of the anteroventral lateral line nerve, like that of the anterodorsal nerve, passes caudally along the lateral surface of the ganglionic complex and fuses with the sensory root of the anterodorsal lateral line nerve before their respective fibers enter the medulla (Fig. 10).
The peripherally directed fibers of the anteroventral lateral line nerve initially form a distinctly recognizable fascicle (Fig. 12A), but as they join the overlying facial sensory and motor fibers within the anterior ganglionic complex to form the hyomandibular trunk, they can no longer be distinguished from the facial fibers. As the hyomandibular trunk separates from the more rostrally coursing superolateral strand, a small but distinct bundle of fibers emerges from the trigeminal sensory ganglion and runs along the medial surface of the hyomandibular trunk for a short distance (Fig. 10) before fusing with it. Thus, the hyomandibular trunk is apparently composed of facial sensory and motor fibers, lateral line fibers, and trigeminal sensory fibers. Application of HRP to the hyomandibular trunk confirms this interpretation, as retrogradely labeled cells occur in the anteroventral lateral line, facial, and trigeminal ganglia, as well as in the facial motor nucleus. A small number of labeled cells were seen in the trigeminal motor nucleus in two of the four cases and probably result from HRP being inadvertently taken up by trigeminal fibers that were transected along with a portion of the mandibular adductor muscle, which overlies the hyomandibular trunk. After exiting the neurocranium, the hyomandibular trunk courses ventrally (Fig. 6) and initially caudally as it passes along the medial surface of the hyomandibular bone. As the trunk approaches the distal end of the hyomandibular bone, it passes through a foramen and emerges onto the lateral surface of the bone. The hyomandibular trunk then passes along the lateral surface of the quadrate bone and continues to pass medial to the preoperculomandibular canal where a small ramule emerges from the trunk to innervate the first neuromast within this canal (Figs. 1A, 6). As the hyomandibular trunk continues ventrally it issues a variable number of small ramules that pass caudally over the surface of the opercular bone to ramify in the overlying skin (Fig. 6). These small ramules seem to innervate the ampullary organs located over and adjacent to the more caudal horizontal segment of the preoperculomandibular canal (Fig. 1B).
After issuing these small ramules, the hyomandibular trunk almost immediately divides into a ventrally directed hyoid ramus and a rostrally directed mandibular ramus (Fig. 5), which again divides into deep and superficial subdivisions (dmd and smd, Fig. 6). As the hyoid ramus continues ventrally and medially, it divides into three main ramules, the most caudal of which ramifies in the skin overlying the ventral half of the opercular region (ch, Fig. 6). The fibers of the caudal ramule seem to innervate a small number of ampullary organs (Fig. 1B) and taste buds located in the more rostroventral segment of the opercular region. The second ramule that arises from the hyoid ramus (mh, Fig. 6) courses medially over the hypobranchial region to ramify in the overlying skin. Again, this ramule seems to innervate ampullary organs (Fig. 6) and taste buds near the ventral midline of the middle segment of the hypobranchial region. The third ramule (rh, Fig. 6) of the hyoid ramus courses rostrally to innervate the hypobranchial muscles and overlying skin, which also contains ampullary organs (Fig. 1B) and taste buds.
Application of HRP to the proximal stump of the base of the hyoid ramus retrogradely labels sensory neurons in the anteroventral, facial and trigeminal rami, as well as in the facial motor nucleus. The centrally directed fibers of these sensory neurons could be traced into the lateral line lobe, facial lobe, and descending trigeminal tract, respectively. The presence of labeled cells within the trigeminal ganglion after application of HRP to the hyoid ramus suggests that this ramus also carries somatic sensory fibers that innervate the skin of the lower cheek and caudal hypobranchial region. This innervation may be provided by the trigeminal sensory bundle that initially joined the hyomandibular trunk near its base (Fig. 10).
The deep mandibular ramus of the anteroventral lateral line nerve contains only lateral line fibers, experimentally confirmed, that innervate the remaining neuromasts (two through eight, Fig. 1A) of the preoperculomandibular canal. This subdivision of the mandibular ramus is located ventral to the articular and dentary bones and runs along the medial surface of the preoperculomandibular canal. Before coming to lie along the lateral surface of the articular bone, the superficial subdivision of the mandibular ramus passes adjacent to the ventrolateral surface of the adductor mandibular muscle for some distance and issues sizable ramules that innervate the superficial neuromasts of the quadratojugal and mandibular pit lines (Figs. 1A, 6), as well as the ampullary organs and taste buds in the skin associated with the middle segment of the preoperculomandibular line. As the superficial subdivision of the combined anteroventral and facial ramus continues rostrally along the lateral surface of the dentary bone, it repeatedly issues ramules (not shown in Fig. 6) that innervate ampullary organs and taste buds in the skin overlying the rostral segment of the preoperculomandibular canal. In Bodian-stained transverse serial sections, it is possible to follow a single ramule as it branches and innervates both ampullary organs and taste buds.
As the superficial subdivision of the combined mandibular ramus continues rostrally, adjacent to the lateral surface of the dentary bone, a laterally and ventrally directed ramule of the mandibular trigeminal ramus fuses with it at approximately the level of the third infraorbital neuromast (Fig. 6). Nearly doubled in size by this fusion, the superficial subdivision of the combined mandibular ramus continues rostrally to the very tip of the lower jaw. As it approaches its termination, it divides into several ramules that innervate ampullary organs and taste buds. It is also possible that the superficial subdivision contains somatic sensory fibers of the trigeminal nerve. After transection and application of HRP to its proximal stump, a short distance from its separation from the deep subdivision, retrogradely labeled cells occur in the anteroventral, facial, and trigeminal ganglia. Thus, it seems that trigeminal fibers join the superficial subdivision of the combined mandibular ramus as it emerges from the hyomandibular trunk, as well as more rostrally where a ramule of the mandibular trigeminal ramus fuses with the superficial subdivision.
Otic lateral line nerve.
The otic is the only lateral line nerve in the channel catfish in which a distinct and separate sensory ganglion cannot be recognized. The nerve bundle termed the otic lateral line nerve is the most rostral bundle to arise from the dorsal surface of the anterodorsal ganglion (Figs. 6, 10). In adult catfish, a slight elevation formed by ganglionic neurons may represent the fusion of the otic ganglion with the anterodorsal lateral line ganglion (Fig. 10). We will treat the bundle of fibers that issues from this elevation as a separate lateral line nerve, based on the fact that an outgroup analysis of bony fishes (Northcutt and Bemis, 1993; Piotrowski and Northcutt, 1996) indicates that an otic lateral line nerve with a distinct and separate sensory ganglion represents the primitive condition for bony fishes. In any case, a single ramus, usually termed the otic ramus, can be traced dorsally and rostrally to exit the neurocranium by means of a separate foramen in the sphenotic bone. After entering the sphenotic bone, the otic ramus turns caudally to course immediately adjacent to the ventromedial wall of the otic lateral line canal (Figs. 1A, 6). It passes under the anterior ramule of the superficial ophthalmic ramus of the anterodorsal lateral line nerve and then continues caudally within the sphenotic, and a short distance rostral to the single neuromast in the otic canal (Fig. 1A) it divides into two ramules (Fig. 6). One innervates the neuromast of the otic canal, whereas the other continues caudally to enter the operculum where it ramifies throughout the dorsal half of the opercular flap. In Bodian preparations, it is possible to trace small bundles of otic fibers into the base of ampullary organs and taste buds. It is clear from these observations that visceral sensory fibers arising from the facial ganglion constitute one fiber population of the otic ramus. It is possible that trigeminal somatic sensory fibers also join the otic ramus as it ramifies over the upper half of the operculum. This region is also innervated by mandibular trigeminal fibers, as well as facial opercular fibers (ope, Figs. 5, 6). Unfortunately, we were not able to label any of these rami with HRP and could not confirm whether or not they include somatic sensory fibers.
Middle lateral line nerve.
The ganglion of the middle lateral line nerve consists of a small number of large cells (no more than 100) with pale cytoplasm. It lies within the neurocranium, ventral to the root of the posterior lateral line nerve (Figs. 11, 13C). The root of the middle lateral line nerve is very short, and its fibers almost immediately join the root of the posterior lateral line nerve (Fig. 11). The single ramus of the middle lateral line nerve exits the neurocranium through a foramen that also houses the root of the glossopharyngeal nerve and the trunk of the vagal nerve. The ramus of the middle lateral line nerve runs rostrally and laterally (Fig. 11), immediately beneath the floor of the otic capsule. As it reaches the lateral edge of the otic capsule, the ramus turns dorsally and divides into anterior and posterior ramules (Fig. 6). The anterior ramule turns dorsally and rostrally to course along the lateral surface of the temporal canal (Fig. 1A). As it approaches the single neuromast within the temporal canal, the anterior ramule divides again, with one branch innervating the neuromast within the temporal canal. The second branch continues a slight distance rostrally and dorsally (Fig. 6) to innervate the two neuromasts of the middle pit line (MP, Fig. 1A). The posterior ramule of the middle lateral line ramus turns caudally and ventrally to enter the most caudal segment of the opercular flap. Unfortunately, we were unable to isolate this nerve in the rather small juvenile catfishes that we used for tracing studies and could not experimentally determine whether this nerve also contains facial and trigeminal sensory fibers.
Posterior lateral line nerve.
The sensory ganglion of the posterior lateral line nerve lies outside the neurocranium, below the caudalmost portion of the otic capsule, which houses the posterior semicircular canal and dorsal to the lateral vagal ganglion (Figs. 11, 13D). Although the posterior lateral line and lateral vagal ganglia are in contact (Fig. 13D), each is distinct because the diameter of the cells of the lateral line ganglion (x = 30.6 μm SE ± 0.59, N50 in the 39-cm-long catfish) is much larger than that (x = 18.6 μm SE ± 0.53, N50) of the lateral vagal ganglion. The cells of the posterior lateral line ganglion also have an extensive pale cytoplasm, as do the cells of the other lateral line ganglia, unlike the scant, darkly staining cytoplasm of the ventral subdivision of the facial ganglion and the lateral vagal ganglion.
The root of the posterior lateral line nerve enters the neurocranium with the trunk of the vagal nerve through the vagal foramen. As the root of the posterior lateral line nerve passes along the ventral and medial surfaces of the crista of the posterior semicircular canal, it lies on the lateral surface of the vagal trunk and is semilunar in shape. As it continues dorsally and rostrally, it passes over the most distal segment of the posterior ramus of the octaval nerve and the ganglion of the middle lateral line nerve where it fuses with the very short root of the latter (Figs. 6, 11). Technically, the combined fibers of the two nerves should be described as the root of the postotic lateral line nerves (pr, Figs. 6, 10, 11). As this root continues rostrally, it passes slightly dorsal to the roots of the glossopharyngeal nerve and then enters the medulla immediately beneath the ventrolateral border of the lateral line lobes (Fig. 11).
The trunk of the posterior lateral line nerve arises from the distal end of the ganglion and runs caudally and dorsally (Figs. 5, 6, 11) to pass over the dorsal surface of the posttemporal bone. A small bundle of fibers emerges directly from the distal end of the ganglion or almost immediately from the trunk (Figs. 6, 11) of the posterior lateral line nerve. The bundle then courses dorsally and laterally to anastomose with the dorsal somatic ramus of the first spinal nerve, then turns rostrally to pass along the lateral surface of the otic capsule. After a short distance, the bundle divides into numerous twigs. One of the larger twigs innervates the first neuromast of the trunk canal (Fig. 1A). Other twigs seem to ramify in the skin, dorsal to the canal, and possibly innervate the first accessory neuromast (AN, Fig. 1A), as well as the ampullary organs in that region.
As the posterior lateral line trunk continues caudally, it passes ventral to the epibranchial trunk muscles, where a second bundle of fibers arises (Figs. 6, 11). This bundle also turns dorsally and rostrally and innervates the second neuromast in the trunk canal (Fig. 1A). The dorsal ramus of the posterior lateral line nerve (Figs. 5, 6, 11) arises from the trunk of the posterior lateral line nerve, approximately one segment more caudally. The dorsal ramus turns dorsally and caudally to innervate the second accessory trunk line, which consists of two neuromasts, the dorsal trunk line, which consists of three neuromasts (Figs. 2, 6) and the ampullary organs associated with these lines.
The posterior lateral line trunk divides into lateral and ventral rami (Figs. 5-8) approximately two body segments after the dorsal ramus arises from the trunk. The lateral ramus innervates the neuromasts of the accessory trunk lines, neuromasts within the trunk canal, neuromasts at the base of the caudal fin, and all of the trunk ampullary organs dorsal to the ventral trunk line. The ventral ramus innervates the neuromasts of the ventral trunk line and all of the trunk ampullary organs ventral to this line of neuromasts (Figs. 2, 3). All of the trunk lateral line organs are innervated by dorsal ramules, ventral ramules, or both, that arise from the lateral and ventral rami (Figs. 6-8). The relationship of these ramules to the spinal nerve rami and ramules of the facial recurrent ramus are very complex, however. Even though the nerves that innervate the trunk represent three different sensory systems (pain, temperature, and touch information by means of the spinal nerves; gustatory information by means of the recurrent facial ramus; and electrosensory and mechanosensory lateral line information by means of the posterior lateral line nerve) all three systems use many of the same ramules as their fibers approach the periphery (Fig. 8). Analysis of Sudan-Black stained trunk nerves in conjunction with Bodian-stained serial transverse sections of the trunk have allowed us to reconstruct the pathways used by each of the three systems.
Fibers of the facial recurrent ramus that innervate taste buds distributed over the trunk initially exit the recurrent ramus by means of segmentally repeated intervertebral ramules (Figs. 6, 8) whose fibers pass through the dorsal root ganglia of the spinal nerves to enter either their dorsal or ventral somatic rami. The gustatory fibers then run toward the periphery in association with the sensory fibers of the spinal nerves (Fig. 8). The combined somatic and gustatory sensory fibers in the somatic rami of the spinal nerves reach the skin surface in one of two ways. The dorsal somatic ramus of each spinal nerve courses toward the surface in the horizontal septum, which separates the epaxial and hypaxial trunk muscles. As the dorsal ramus approaches the medial surface of the lateral ramus of the posterior lateral line nerve, it divides into dorsal and ventral ramules (dra and vra, Fig. 8). Each of these ramules then turns to course within the myoseptum, and fibers of both ramules ramify among the muscle fibers of a single myotome. However, each ramule innervates the skin in a different manner. Fibers of the dorsal ramule continue within the myoseptum until they reach the inner surface and ramify. As the fibers of the dorsal ramule ramify, many seem to anastomose with the branching fibers of the dorsal ramule (dr) of the lateral ramus of the posterior lateral line nerve. The lateral ramus issues a single dorsal and ventral ramule per trunk segment, and these ramules course dorsally or ventrally, immediately beneath the skin, above the myotome. As each ramule moves away from the lateral ramus, it branches repeatedly, and a single ramule may span as many as four trunk segments. Although anastomoses occur only at the skin surface between the dorsal ramule of the lateral ramus and the dorsal ramule of the dorsal somatic ramus, the ventral ramule (vra) of the dorsal spinal nerve anastomoses almost immediately with the ventral ramule (vr) of the lateral ramus (Fig. 8) so that somatic sensory, lateral line, and gustatory fibers run in the same fiber bundles as they ramify just beneath the surface of the skin. A similar relationship seems to characterize the lateral ramule (le) of the ventral somatic ramus and the ventral ramule (vr) of the ventral ramus (v) of the posterior lateral line nerve.
As the lateral ramus of the posterior lateral line nerve reaches the caudal end of the trunk, it assumes a new set of relationships with the facial recurrent ramus and the spinal nerves as all these nerves enter the caudal fin (Fig. 9). As the spinal cord approaches the base of the caudal fin, it is deflected dorsally to form a neurohaemal organ, the urophysis. At this point of transition, a terminal dorsal somatic ramus (tds) and a terminal ventral somatic ramus (tvs) arise from the spinal cord (Fig. 9B). The terminal dorsal somatic ramus parallels the caudal continuation of the urophysis and is joined by the facial recurrent ramus at the point of origin of the terminal dorsal somatic ramus (Fig. 9A). In contrast, the terminal ventral somatic ramus continues caudally at the same dorsoventral level as it arose from the spinal cord (Fig. 9B). Both the terminal dorsal and terminal ventral somatic rami continue caudally to the bases of the fin rays (lepidotrichia). At this point, each ramus divides into dorsal and ventral ramules that form a dorsal arch and a ventral arch (Fig. 9B). The dorsal arch is formed by the ventral ramule of the terminal dorsal somatic ramus and the dorsal ramule of the terminal ventral somatic ramus, whereas the ventral arch is formed by the ventral ramule of the terminal ventral somatic ramus and by four more rostral ventral somatic rami (vsr, Fig. 9A). Neural elements arise from each arch and continue within the center of each fin ray. Each neural element is composed of somatic sensory fibers as well as special visceral (gustatory) sensory fibers. These sensory fibers also will be joined by the electroreceptive fibers of the lateral ramus of the posterior lateral line nerve (Fig. 9). As the lateral ramus approaches the base of the caudal fin, it divides into three or four ramules that continue caudally just beneath the skin. As these ramules fan out dorsoventrally, some of their fibers innervate the vertical line of neuromasts at the base of the caudal fin (VLN, Fig. 2B). The remaining fibers of the ramules of the lateral ramus of the posterior lateral line nerve continue caudally and medially to anastomose with the neural elements within the fin rays (Fig. 9A), and these lateral line fibers innervate the ampullary organs located on the caudal fin (Fig. 3B).
DISCUSSION
Our observations on the lateral line system generally corroborate earlier studies of this system in other catfishes but do differ in several details. These similarities and difference will be discussed first. We will then discuss the homology and polarity (e.g., are various features primitive or derived) of the neuromast lines, their associated ampullary fields, and their innervation. Finally, we will discuss some of the developmental implications of the distribution of neuromasts and ampullary organs, as well as the significance of the anastomoses of the peripheral trunk rami of the facial, spinal and lateral line nerves.
Earlier studies
The first description of ampullary organs and external taste buds in catfish seems to be Wright's study (1884a) on the cutaneous sense organs of the white catfish, Ictalurus (Amiurus) catus. He recognized the superficial similarity between the catfish ampullary organs and the primitive electroreceptive ampullae of Lorenzeni, i.e., both organs occur as pits in the skin in sharks and as so-called nerve sacs in sturgeons, but he had no idea of the function of either. Although all of these organs have proved to be electroreceptors (reviewed in Kalmijn, 1974; Finger, 1986; Zakon, 1988), the fact that catfish ampullary organs are not homologous to primitive electroreceptors did not emerge until much later (McCormick, 1982; Bullock et al., 1983; Zakon, 1988). Wright did describe the external taste buds in catfishes and realized their homology to the pharyngeal taste buds of other vertebrates but focused on their tactile function, apparently because of the larger numbers of these organs on the barbels. Wright also recognized both canal and superficial neuromasts in catfish but did not describe the canals in detail and recognized only the quadratojugal and ventral trunk lines.
The first detailed description of the canal and superficial neuromasts in catfish is the study by Herrick in 1901 on the cutaneous sense organs of the black bullhead catfish, Ameirus (Ictalurus) melas. Herrick correctly identified the preotic canals, as well as the number of neuromasts in each. The only difference in the number of preotic canal neuromasts in A. melas and I. nebulosus is that A. melas seems to possess four superficial neuromasts rather than two at the rostral end of the infraorbital line. Herrick also noted that the otic and temporal canals each contain only a single neuromast, but he did not realize that each of these organs and their canals arose from different placodes and assumed that they were part of the lateral trunk line. Although Herrick did not describe the superficial neuromast lines in detail, his map of their distribution, his Figure 14, is remarkably accurate considering that he used only a hand lens. He correctly identified all of the cephalic pit lines, as well as the dorsal and ventral trunk lines. Although it is clear that he recognized some of the accessory trunk neuromasts, it is not clear that he realized that these neuromasts occur in vertical lines.
Herrick, like Wright (1884a), realized that catfish possess an additional class of lateral line receptors, the ampullary organs or small pit organs and that these organs occur on both the head and trunk, but he did not attempt to map them. Peters et al. (1974) produced the first map of catfish ampullary organs when they and Roth (1968, Roth 1969) realized that catfish could detect electric fields and might, therefore, use these organs to detect each other. The ampullary map produced by Peters et al. was based on a single individual whose length was between 18 and 21 cm. The ampullary organs were visualized macroscopically by submerging freshly killed animals in 1% nitric acid, which revealed the pore openings of the organs as small black holes. The authors noted that the skin of each of their specimens was damaged and that it was necessary to estimate the number of receptors based on counts of the receptors histologically from 1 cm2 skin samples. Unfortunately, approximately half of the skin surface of the mapped individual was missing, and the map conveys little information about differences in the distribution of the receptors. Likewise, their estimates of the number of receptors (2,500 to 4,000) is problematic, as the considerably smaller specimen (6.8 cm total length) that we measured also had approximately 4,000 receptors. It would be interesting to determine the variation in the number of receptors in several individuals of the same length and to determine whether the receptors increase in number with body length.
We arrived at our understanding of the innervation of the lateral line receptors in two phases: early descriptions of the nerves (Wright, 1884b; Herrick, 1901), followed by a long period, over 50 years, in which no studies were done, and a second phase of experimental studies (Knudsen, 1976a,Knudsen, 1976b; Finger, 1976; Finger and Bullock, 1982; Tong and Finger, 1983; Finger and Tong, 1984; New and Singh, 1994) that focused primarily on the central lateral line pathways, with little attention to peripheral organization. Wright's initial description (1884b), primarily based on gross dissection of the cranial nerves, is remarkable for its accuracy regarding nerve roots, ganglia, and major rami that innervate the lateral line and gustatory systems. Wright realized that the facial (the preotic lateral line ganglia were included as part of the facial nerve) and trigeminal nerves were fused, and he was the first to recognize and name the inferomedial and superolateral strands. Without doubt, his observation of the fiber exchange between the two strands was one of his remarkable observations.
In 1901, Herrick published his classic study on the cranial nerves of catfish, based on serially sectioned material, a laborious reconstruction of the course of these nerves relative to the lateral line receptors, and a reconstruction of the anterior ganglionic complex. Our observations corroborate most of Herrick's conclusions with several exceptions. Herrick believed that the facial superficial ophthalmic ramus, our superficial ophthalmic ramus of the anterodorsal lateral line nerve, consisted only of lateral line fibers. In a sense he was correct as this ramus does originate primarily from a distinct anterodorsal ganglion. However, it is joined by somatic and visceral sensory fibers derived from the trigeminal and facial ganglia, respectively.
Herrick's error regarding the composition of the superficial ophthalmic ramus may have resulted in a second error. Herrick termed the profundal ramus of Wright's and our description a trigeminal superficial ophthalmic ramus. We suspect he reached this conclusion because a trigeminal superficial ophthalmic ramus does innervate the rostral skull roof in sharks (Norris and Hughes, 1920) and primitive ray-finned fishes such as Amia (Norris, 1925). However, the ramus that both Herrick (1901) and we identify as carrying lateral line fibers also carries trigeminal sensory fibers. Therefore, there is no basis for recognizing a second ramus that consists of trigeminal somatic sensory fibers (e.g., Herrick's superficial ophthalmic ramus of the trigeminal nerve). We believe that Wright's interpretation (1884b) is correct because the ramus that we recognize as the profundal ramus does innervate the snout as the deep ophthalmic or profundal ramus does in other vertebrates. Unfortunately, it is not possible to determine whether Wright or Herrick was correct based on the fiber composition of each ramus. Although it is unlikely, it is even possible that our profundal ramus is a combination of part of the trigeminal superficial ophthalmic ramus and the profundal ramus of other fishes. A definitive answer awaits developmental observations, as a profundal nerve should arise from a distinct placode that initially forms a sensory ganglion that may or may not secondarily fuse with the trigeminal ganglion (Northcutt, 1992a; Northcutt and Brändle, 1995).
Wright and Herrick also disagreed regarding the nature of the fibers that form the inferomedial and superolateral strands. Wright believed that both strands were composed of both facial and trigeminal fibers, whereas Herrick believed that the inferomedial and superolateral strands consisted only of facial and trigeminal fibers, respectively. It is impossible to determine which interpretation is correct based on the application of any tracer to the peripheral rami, because most rami carry both facial and trigeminal fibers. We are inclined to support Wright's interpretation, based on the extensive exchange of fibers that occurs between strands just before their forming the maxillary and mandibular rami. This fiber exchange was first noted by Wright (1884b) and we confirm his observations. As far as we have been able to tell, Herrick (1901) did not comment on this fiber exchange between strands. It should be possible to resolve the composition of these strands by injecting a tracer into the facial lobe and examining both strands for labeled fibers. If Wright is correct, labeled fibers should occur in both strands. If Herrick is correct, labeled fibers should be restricted to the inferomedial strand.
Our reconstruction of the anterior ganglionic complex agrees closely with Herrick's reconstruction. His description of the relative positions of the facial, lateral line and trigeminal ganglia within the complex seems essentially correct. Unfortunately, the symbols for the various ganglia he mentions in the legend for his Figure 2 (his reconstruction) do not appear on the figure itself. In our reconstruction, we have not included Herrick's accessory maxillary nerve. We agree with Herrick that the fibers of this “nerve” do arise from the inferomedial and superolateral strands, but in our preparation the fibers from each strand almost immediately join the buccal ramus before its division into inner and outer segments. Herrick's description and figures indicate that the accessory maxillary forms a distinct bundle that parallels the outer buccal ramus. These observational differences may be due to either individual or interspecific variation. In Herrick's reconstruction, a bundle of facial fibers joins the otic ramus, and a trigeminal bundle passes dorsal to this ramus. Although we believe that the otic ramus contains facial fibers, because we traced fibers from the otic ramus that innervate taste buds, we never saw a distinct trigeminal fascicle pass over the otic ramus as indicated by Herrick. We did see a distinct trigeminal fascicle join the anterior ramule; however, before the ramule passes over the otic ramus. Again, these differences may be due to errors in reconstruction or to inter- or intraspecific variation. In any case, both the anterior ramule and the otic ramus appear to contain facial and trigeminal sensory fibers in addition to their lateral line fibers.
Phylogenetic considerations
All of the features of the lateral line canals of the channel catfish are primitive for ray-finned fishes with the exception of the following derived features: (1) the supraorbital and infraorbital canals are not continuous rostrally due to the commissural canal being reduced to a line of two superficial neuromasts; (2) the first neuromast of the anterior pit line is enclosed in a canal that is a caudal extension of the supraorbital canal; (3) the peroperculomandibular canal caudally joins the postotic canals (Fig. 1A) rather than ending slightly ventral to these canals; (4) catfish have lost the supratemporal commissural line of neuromasts that is usually enclosed in a canal interconnecting the posttemporal canals; (5) the pit line pattern has been altered by the loss of the horizontal, vertical, gular, and posterior pit lines; (6) a reduced dorsal trunk line and the elaboration of the accessory trunk lines. As already noted, the presence of cephalic and trunk ampullary organs is also a derived feature in catfishes.
In addition, many aspects of the innervation of the cephalic lateral line and other receptors are also derived features: (1) the probable loss of a distinct trigeminal superficial ophthalmic ramus; (2) a probable reversal in the positions of the otic ramus and the anterior ramule; (3) the absence of a mandibular facial ramus; (4) the loss of a supratemporal lateral line nerve; (5) the presence of a recurrent facial ramus and facial fibers within almost all other branchiomeric nerves due to the presence of external taste buds; (6) unique divisions of the maxillary and mandibular rami associated with barbel development; and (7) fusion of the ganglia of the facial, preotic lateral line, and trigeminal nerves to form the anterior ganglionic complex. Many, if not most, of these changes in cephalic lateral line and receptor innervation seem to be correlated with the development of external taste buds rather than the development of electroreceptors.
Developmental considerations
The distribution of electroreceptors and the close association of the posterior lateral line nerves with the facial recurrent ramus and spinal nerves raise important developmental questions, beyond the obvious questions of how external taste buds are developed and maintained. As Herrick (1901) initially suggested, the ampullary organs of catfish are not randomly distributed on the head. With the exception of the hypobranchial ampullary field, all of the other ampullary organs are centered on the lateral line canals. Even though the ampullary organs of catfish are not homologous to those of axolotls, the ampullary organs in axolotls are even more restricted to areas adjacent to the lateral lines (Northcutt, 1992a). The development of ampullary organs in axolotls has been extensively documented (Smith et al., 1988, Smith et al., 1990; Northcutt et al., 1994, Northcutt et al., 1995). In these animals, the lateral line placodes initially elongate to form sensory ridges (the posterior lateral line placode, which migrates down the trunk, is an exception). The primordia of the neuromasts form within a central strip of the sensory ridge flanked by the ampullary primordia. The distribution of ampullary organs in the channel catfish suggests that these organs may develop in the same way as in axolotls. However, it is possible that the ampullary organs of catfish are induced directly from general ectoderm by the sprouting of lateral line fibers (Vischer et al., 1989). Resolution of how these receptors are induced and how they differentiate are critical for understanding how lateral line systems change through time.
The close association of the trunk fibers (facial, lateral line, and spinal nerves) that innervate the skin and its specialized sensory receptors suggests that the nerve fibers of one of these systems may establish the distribution pattern for the other fibers. Fibers of the recurrent facial ramus almost certainly do not establish this pattern, despite the ramus's issuing segmental invertebral ramules, because there is also a close association between the lateral line and spinal fibers in zebrafish, for which no external taste buds occur on the trunk (unpublished observations, RGN).
Partial ablation of trunk neural crest in axolotls disrupts the regular development of trunk neuromasts in axolotls (Smith et al., 1994) and suggests that neural crest or its derivatives may be involved in patterning trunk nerves. The migrating posterior lateral line placode deposits neuromast primordia at regular intervals until it reaches the point on the trunk where neural crest has been removed. At this point, the placode stops depositing neuromast primordia but continues to migrate. When the placode again reaches a normal trunk segment, it resumes depositing neuromast primordia. Smith et al. (1994) suggested that the migrating placode was receiving clues for the deposition of sensory primordia from myosepta or from spinal nerve fibers that run within the myosepta. In either case, the spinal fibers and their associated glial elements are derived from neural crest, as is much of the connective tissue that forms the myosepta. The presence of a canal neuromast and its associated innervation in each trunk segment is certainly suggestive. It is also possible that the recurrent facial ramus uses similar clues to issue intervertebral ramules. The embryos of axolotls, catfish, and zebrafish offer fertile ground to test this model in more detail.
The neural crest origin of spinal nerves and their intimate relationship to neuromasts and the lateral line fibers that innervate them may explain another set of developmental observations. When neural crest has been labeled in several species of fish, labeled cells have been detected near neuromasts (Lamers et al., 1981) or within neuromasts (Collazo et al., 1994) later in development. These observations have resulted in the claim that neuromasts have a dual embryonic origin from neural crest and lateral line placodes (Collazo et al., 1994). The intimate relationship of lateral line and spinal ramules suggests another interpretation, however. It is possible that some fraction of the glial cells that ensheath fibers of the lateral line ramules arise from neural crest as, apparently, do all of the glial cells of the spinal nerves. If this is the case, it is possible that migrating Schwann cells of neural crest origin use both spinal and lateral line fibers and ensheath both. In this context, it may be significant that Collazo et al. (1994) claim that neuromasts derived from placodes appeared to differentiate earlier than neuromasts derived from neural crest. The unusual relationship of fibers of spinal nerve origin, as well as those of cranial nerve origin, suggests that there may also be very complicated interactions between neural crest and placodal derivatives.
Acknowledgements
We were fortunate to have had the technical assistance of Mariola Milik. We thank Georg Striedter for the loan of a set of Bodian-stained transverse sections of the brain of a channel catfish, which allowed us to photograph the section in Figure 11A. Sue Commerford provided literature retrieval, word processing, and other support services, and Mary Sue Northcutt assisted in numerous phases of the study. P.H.H. was supported in part by NRSA training grant and R.G.N. received support from a NIH research grant.




