Immunohistochemical localization of prostaglandin EP3 receptor in the rat nervous system
Abstract
The prostaglandin EP3 receptor (EP3R) subtype is believed to mediate large portions of diverse physiologic actions of prostaglandin E2 in the nervous system. However, the distribution of EP3R protein has not yet been unveiled in the peripheral or central nervous systems. The authors raised a polyclonal antibody against an amino-terminal portion of rat EP3R that recognized specifically the receptor protein. In this study, immunoblotting analysis with this antibody showed several immunoreactive bands with different molecular weights in rat brain extracts and in membrane fractions of recombinant EP3R-expressing culture cells, and treatment with N-glycosidase shifted those immunoreactive bands to an apparently single band with a lower molecular weight, suggesting that EP3R proteins are modified posttranslationally with carbohydrate moieties of various sizes. The authors performed immunohistochemical investigation of EP3R in the rat brain, spinal cord, and peripheral ganglia by using the antibody. EP3R-like immunoreactivity was observed in many and discrete regions of the rostrocaudal axis of the nervous system. The signals were particularly strong in the anterior, intralaminar, and midline thalamic nuclear groups; the median preoptic nucleus; the medial mammillary nucleus; the superior colliculus; the periaqueductal gray; the lateral parabrachial nucleus; the nucleus of the solitary tract; and laminae I and II of the medullary and spinal dorsal horns. Sensory ganglia, such as the trigeminal, dorsal root, and nodose ganglia, contained many immunopositive neurons. Neuronal cells in the locus coeruleus and raphe nuclei exhibited EP3R-like immunoreactivity. This suggests that EP3R plays regulatory roles in the noradrenergic and serotonergic monoamine systems. Autonomic preganglionic nuclei, such as the dorsal motor nucleus of the vagus nerve, the spinal intermediolateral nucleus, and the sacral parasympathetic nucleus, also contained neuronal cell bodies with the immunoreactivity, implying modulatory functions of EP3R in the central autonomic nervous system. The characteristic distribution of EP3R provides valuable information on the mechanisms for various physiologic actions of prostaglandin E2 in the central and peripheral nervous systems. J. Comp. Neurol. 421:543–569, 2000. © 2000 Wiley-Liss, Inc.
Prostaglandin (PG) E2 is known to have various physiologic functions in the nervous system, such as fever induction (Stitt, 1986; Blatteis and Sehic, 1998), pain modulation (Ferreira, 1972; Ferreira et al., 1973, Ferreira et al., 1978), regulation of vasopressin and luteinizing hormone-releasing hormone secretion (Vilhardt and Hedqvist, 1970; Ojeda et al., 1982), regulation of food intake (Scaramuzzi et al., 1971), and sleep-wake regulation (Onoe et al., 1992; Hayaishi and Matsumura, 1995). PGE2 exerts these diverse actions through specific cell-surface receptors. The PGE receptors were divided pharmacologically into four subtypes (EP1, EP2, EP3, and EP4) on the basis of their responses to various agonists and antagonists. Molecular cloning and biochemical approaches revealed that they belong to a rhodopsin-type receptor superfamily and are coupled to various signal-transduction systems (for review, see Negishi et al., 1995). To clarify the mechanisms for those PGE2 actions, several histochemical studies were performed in the central nervous system (CNS). However, most of the studies focused on cyclooxygenases—rate-limiting enzymes in the biosynthesis of PGs (Breder et al., 1992, Breder et al., 1995)—and demonstrated that cyclooxygenase-2 was induced in discrete populations of neuronal cells by synaptic stimuli (Yamagata et al., 1993). Those studies contributed to an explanation of the PG production mechanism in the CNS, whereas histochemical approaches to the PGE receptors have almost never been performed.
Among the PGE receptor subtypes, the EP3 receptor (EP3R) has been the best characterized and has been reported to be involved in contraction of the uterus (Krall et al., 1984), inhibition of gastric acid secretion (Chen et al., 1988), lipolysis in the adipose tissue (Richelsen and Beck-Nielsen, 1984), and sodium and water reabsorption in the kidney tubules (Garcia-Perez and Smith, 1984). These various actions of EP3R have been thought to be induced by inhibition of adenylate cyclase or stimulation of Ca2+ mobilization (Negishi et al., 1995). In addition to peripheral actions, EP3R also has been shown to have physiologic roles in the nervous system, such as fever induction (Ushikubi et al., 1998), pain modulation (Kumazawa et al., 1993; Minami et al., 1994; Oka et al., 1997), and regulation of the autonomic nervous system (Mantelli et al., 1991; Molderings et al., 1992, Molderings et al., 1994; Yokotani et al., 1996; Reinheimer et al., 1998; Spicuzza et al., 1998). However, the neuronal mechanisms for the diverse functions of EP3R remain obscure. Details of the distribution of the receptor in the nervous system would provide a great deal of information on those mechanisms. Although several reports have indicated PGE2 binding sites in the mammalian brain using a radiolabelled ligand (Watanabe et al., 1988; Matsumura et al., 1990, Matsumura et al., 1992) and an anti-PGE2 antibody (Fujimoto et al., 1992), it has not yet been identified which subtypes of PGE receptors are expressed in those sites.
We previously showed the distribution of mRNA for EP3R in the mouse nervous system by using in situ hybridization (Sugimoto et al., 1994). Recently, we produced a polyclonal antibody specific to rat EP3R and revealed the immunocytochemical localization of the receptor protein in the rat hypothalamus (Nakamura et al., 1999). In the current study, we examined in detail the distribution of immunoreactivity with the antibody against EP3R and mapped the function sites of EP3R in the rostrocaudal axis of the rat central and peripheral nervous systems.
Abbreviations
-
- 3V
-
third ventricle
-
- 4V
-
fourth ventricle
-
- ac
-
anterior commissure
-
- ACo
-
anterior cortical amygdaloid nucleus
-
- AD
-
anterodorsal thalamic nucleus
-
- AH
-
anterior hypothalamic area
-
- Am
-
ambiguus nucleus
-
- AM
-
anteromedial thalamic nucleus
-
- AP
-
area postrema
-
- APt
-
anterior pretectal nucleus
-
- Aq
-
aqueduct
-
- Ar
-
arcuate nucleus
-
- AT
-
anterior tegmental nucleus
-
- AV
-
anteroventral thalamic nucleus
-
- BL
-
basolateral amygdaloid nucleus
-
- BM
-
basomedial amygdaloid nucleus
-
- CA1–CA3
-
fields CA1–3 of Ammon's horn
-
- Ce
-
central amygdaloid nucleus
-
- Cf
-
cuneiform nucleus
-
- CL
-
central lateral thalamic nucleus
-
- CM
-
central medial thalamic nucleus
-
- CP
-
caudate-putamen
-
- Cu
-
cuneate nucleus
-
- CVL
-
caudoventrolateral reticular nucleus
-
- DG
-
dentate gyrus
-
- DH
-
spinal dorsal horn
-
- DMc
-
compact part of the dorsomedial hypothalamic nucleus
-
- DMd
-
dorsal part of the dorsomedial hypothalamic nucleus
-
- DMV
-
dorsal motor nucleus of the vagus nerve
-
- DpG
-
deep gray layer of the superior colliculus
-
- DpW
-
deep white layer of the superior colliculus
-
- DR
-
dorsal raphe nucleus
-
- DT
-
dorsal tegmental nucleus
-
- EPi
-
endopiriform nucleus
-
- f
-
fornix
-
- fr
-
fasciculus retroflexus
-
- Gia
-
alpha part of the gigantocellular reticular nucleus
-
- Giv
-
ventral part of the gigantocellular reticular nucleus
-
- GP
-
globus pallidus
-
- Hy
-
hypoglossal nucleus
-
- IC
-
inferior colliculus
-
- IML
-
intermediolateral nucleus
-
- InG
-
intermediate gray layer of the superior colliculus
-
- InW
-
intermediate white layer of the superior colliculus
-
- IOC
-
inferior olivary complex
-
- IP
-
interpeduncular nucleus
-
- LA
-
lateral amygdaloid nucleus
-
- LC
-
locus coeruleus
-
- LD
-
laterodorsal thalamic nucleus
-
- LG
-
lateral geniculate nucleus
-
- Li
-
linear nucleus of the raphe
-
- LP
-
lateral posterior thalamic nucleus
-
- LPB
-
lateral parabrachial nucleus
-
- LS
-
lateral septal nucleus
-
- LV
-
lateral ventricle
-
- MDl
-
lateral part of the mediodorsal thalamic nucleus
-
- Me
-
medial amygdaloid nucleus
-
- MG
-
medial geniculate nucleus
-
- MHb
-
medial habenular nucleus
-
- Mm
-
median part of the medial mammillary nucleus
-
- MnPO
-
median preoptic nucleus
-
- MnR
-
median raphe nucleus
-
- MPB
-
medial parabrachial nucleus
-
- MPO
-
medial preoptic area
-
- mt
-
mammillotegmental tract
-
- oc
-
optic chiasm
-
- Op
-
optic nerve layer of the superior colliculus
-
- OPt
-
olivary pretectal nucleus
-
- OT
-
nucleus of the optic tract
-
- P
-
pontine nuclei
-
- Pa
-
paraventricular hypothalamic nucleus
-
- PAG
-
periaqueductal gray
-
- PC
-
paracentral thalamic nucleus
-
- Pe
-
periventricular hypothalamic nucleus
-
- Pef
-
perifornical nucleus
-
- Pf
-
parafascicular thalamic nucleus
-
- PH
-
posterior hypothalamic area
-
- Pir
-
piriform cortex
-
- PLCo
-
posterolateral cortical amygdaloid nucleus
-
- PMCo
-
posteromedial cortical amygdaloid nucleus
-
- PMd
-
dorsal part of the premammillary nucleus
-
- PnR
-
pontine raphe nucleus
-
- Po
-
posterior thalamic nuclear group
-
- PRa
-
alpha part of the parvicellular reticular nucleus
-
- PS
-
parastrial nucleus
-
- PT
-
paratenial thalamic nucleus
-
- PTR
-
pontine tegmental reticular nucleus
-
- PV
-
paraventricular thalamic nucleus
-
- R
-
red nucleus
-
- Re
-
reuniens thalamic nucleus
-
- Rh
-
rhomboid thalamic nucleus
-
- RMg
-
raphe magnus nucleus
-
- ROb
-
raphe obscurus nucleus
-
- RPa
-
raphe pallidus nucleus
-
- RRF
-
retrorubral field
-
- RS
-
retrosplenial cortex
-
- RVL
-
rostroventrolateral reticular nucleus
-
- SCh
-
suprachiasmatic nucleus
-
- SF
-
septofimbrial nucleus
-
- SG
-
suprageniculate thalamic nucleus
-
- Sm
-
submedius (or gelatinosus) thalamic nucleus
-
- SM
-
supramammillary nucleus
-
- SNc
-
compact part of the substantia nigra
-
- SNr
-
reticular part of the substantia nigra
-
- SO
-
supraoptic nucleus
-
- SOC
-
superior olivary complex
-
- Sol
-
nucleus of the solitary tract
-
- SP
-
sacral parasympathetic nucleus
-
- SPf
-
subparafascicular thalamic nucleus
-
- SubC
-
subcoeruleus nucleus
-
- SubI
-
subincertal nucleus
-
- SuG
-
superficial gray layer of the superior colliculus
-
- VA
-
ventral anterior thalamic nucleus
-
- Vc
-
caudal part of the spinal trigeminal nucleus
-
- VH
-
spinal ventral horn
-
- Vi
-
interpolar part of the spinal trigeminal nucleus
-
- VL
-
ventral lateral thalamic nucleus
-
- VM
-
ventral medial thalamic nucleus
-
- Vmo
-
motor trigeminal nucleus
-
- Vo
-
oral part of the spinal trigeminal nucleus
-
- Vp
-
principal sensory trigeminal nucleus
-
- VPL
-
ventral posterolateral thalamic nucleus
-
- VPM
-
ventral posteromedial thalamic nucleus
-
- VT
-
ventral tegmental nucleus
-
- Xi
-
xiphoid thalamic nucleus
-
- ZI
-
zona incerta
-
- Zo
-
zonal layer of the superior colliculus
MATERIALS AND METHODS
Antibody production and purification
The production, purification, and characterization of the antibody against rat EP3R have been reported previously (Nakamura et al., 1999). Briefly, antisera were raised in rabbits by immunization with a bacterially expressed glutathione-S-transferase fused with an N-terminal part of rat EP3R (residues 2–28; A G V W A P E H S V E A H S N Q S S A A D G C G S V S; single-letter amino acid code), which is a common portion to known three splice variants, EP3α, EP3β, and EP3γ. A polyclonal antibody was purified from the antisera with a Protein G Superose column (Pharmacia, Uppsala, Sweden) followed by affinity purification with an AffiGel 15 column (BioRad, Hercules, CA) conjugated with a synthetic peptide (TANA Laboratories, Houston, TX) corresponding to residues 2–28 of rat EP3R. The antibody eluted from the affinity column with 0.1 M glycine-HCl, pH 2.5, showed high reactivity and specificity to rat EP3R and was used in this study.
Immunopurification of EP3R protein
Because the content of endogenous EP3R protein in rat brains was too low to be detected with immunoblotting analysis, immunopurification of the receptor protein was carried out as described previously (Nakamura et al., 1999). In brief, rat brains were homogenized with 9 volumes of 50 mM Tris-HCl, pH 7.4, containing 0.5% Triton X-100, 5 mM ethylenediaminetetraacetic acid, 0.2 mM phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 0.1 mM benzamidine followed by centrifugation at 100,000 × g for 1 hour. The supernatant was applied to an AffiGel 10 column (BioRad) conjugated with the affinity-purified antibody (1.6 mg antibody/1 ml gel). After a vigorous wash with 1 M NaCl containing 1% Tween 20, bound protein was eluted from the column with 0.1 M glycine-HCl, pH 2.5, containing 1% Tween 20, and the eluate was subjected to deglycosylation.
Deglycosylation and immunoblotting
Membrane fractions from rat EP3β receptor-expressing COS-7 green monkey kidney cells were prepared by using the same method described previously (Nakamura et al., 1999). The eluate from the antibody column (180 ng protein) or membrane fractions of the COS-7 cells (77 μg protein) were boiled for 5 minutes in 75 μl of 1% sodium dodecyl sulfate (SDS) solution containing 1% 2-mercaptoethanol. After it was placed at room temperature for 10 minutes, the solution was mixed with 25 μl of 0.2 mM potassium phosphate buffer, pH 7.0, containing 2% Nonidet P-40. To 50 μl of this sample, 10 μl of 200 units/ml N-glycosidase F (Boehringer Mannheim, Indianapolis, IN) were added, and the mixture was incubated at 37°C for 2 hours. Instead of the enzyme, the same volume of water was added to untreated samples. After the incubation, all the protein samples were subjected to electrophoresis in a 10% polyacrylamide gel with SDS and transferred onto a polyvinylidene difluoride membrane. After blocking with 3% skim milk, the membrane was incubated with 1.4 μg/ml rabbit anti-rat EP3R antibody and then with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) antibody (dilution, 1:2,000; BioSource International, Camarillo, CA) followed by detection with an enhanced chemiluminescence system (Amersham, Buckinghamshire, United Kingdom). In a detailed analysis of electrophoretic mobility, a 12.5% polyacrylamide gel containing 6 M urea was used for the electrophoresis, and the horseradish peroxidase activity was detected with a tetramethylbenzidine substrate kit (Vector Laboratories, Burlingame, CA).
Immunohistochemistry
Ten male Sprague-Dawley rats (body weight, 200–400 g; SLC Japan, Hamamatsu, Japan) were deeply anesthetized with intraperitonial administration of pentobarbital (50 mg/kg body weight) and were fixed by transcardial perfusion with 300 ml of a cold fixative containing 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. The brain and spinal cord were removed, cut into several blocks, postfixed in the same fixative at 4°C for 8 hours, and then saturated with 0.1 M phosphate buffer containing 25% (weight/volume) sucrose at 4°C for 48 hours. The blocks were cut into 40-μm-thick frontal sections on a cryostat at −20°C. The sections were collected consecutively into six bottles; six series of sections were obtained from each rat. The trigeminal, dorsal root, nodose, superior cervical, and stellate ganglia also were removed, postfixed, cryoprotected, and cut into 20-μm-thick frozen sections. The sections of the ganglia were mounted onto poly-L-lysine-coated slides, air-dried briefly, and then rehydrated in 50 mM phosphate-buffered saline (PBS), pH 7.3.
The sections were incubated overnight at room temperature with 1 μg/ml affinity-purified rabbit antibody to rat EP3R in PBS containing 0.3% Triton X-100, 0.25% λ-carrageenan, and 0.5% normal donkey serum. After a rinse in PBS containing 0.3% Triton X-100, the sections were incubated for 1 hour with 10 μg/ml biotinylated donkey antibody to rabbit IgG (Chemicon, Temecula, CA). The sections were rinsed again and reacted for 1 hour with avidin-biotin-peroxidase complex (1:50; Elite ABC Kit; Vector Laboratories). After a thorough wash of the sections in PBS, bound peroxidase finally was visualized by incubation for 20–40 minutes with 0.02% 3,3′-diaminobenzidine tetrahydrochloride (Dojindo, Kumamoto, Japan) and 0.001% hydrogen peroxide in 50 mM Tris-HCl, pH 7.6. In control experiments, the primary antibody was preincubated with a 16-fold (molar ratio) excess amount of the fusion protein used as the immunogen for 1 hour at room temperature prior to the incubation of sections. For the study of cytoarchitecture, one of the rostrocaudal series of the immunostained sections was counterstained with cresyl violet. The anatomic nomenclature of Paxinos and Watson (1998) was adopted in most regions of the nervous system. Data and figures are from five representative rats. No significant differences in immunostaining patterns were found between animals. All experimental procedures in the current study were approved by the Animal Care and Use Committee at the Graduate School of Biostudies at Kyoto University, Japan.
RESULTS
Characterization of EP3R protein
In our previous study with an antibody to rat EP3R (Nakamura et al., 1999), we performed immunoblotting of the rat brain, kidney, and EP3R-expressing COS-7 cells to characterize the antibody and detected several immunoreactive bands with different molecular weights, all of which were absorbed by preincubation of the antibody with the immunogen, indicating that the antibody specifically recognizes EP3R protein. The heterogeneity of the immunoreactive bands was attributed to various sizes of carbohydrate moieties that were added posttranslationally onto the receptor protein molecules, because rat EP3R has two potential sites for N-linked glycosylation in the extracellular domains (Takeuchi et al., 1993, Takeuchi, et al., 1994; Kitanaka et al., 1996). Here, we examined this possibility. Immunoblotting analysis was performed to detect glycosylated EP3R protein in the rat brain or in the COS-7 cells transiently expressing rat EP3β receptor (Fig. 1A). Because we could not detect any immunoreactivity in crude extracts of rat brains (Nakamura et al., 1999), the extracts were applied to an anti-EP3R antibody-conjugated column to increase the concentration of the endogenous receptor protein. In the eluate from the column (Fig. 1A, lane 1), the antibody showed major immunoreactive bands at around 50 kDa (Fig. 1A, arrow) and a minor immunoreactive band at about 39 kDa. Treatment of the eluate with N-glycosidase shifted these immunoreactive bands to an apparently single band at about 33 kDa (Fig. 1A, lane 2, arrowhead). In membrane fractions of rat EP3β receptor-expressing COS-7 cells, major and broad immunoreactive bands were detected at 60–100 kDa, and three minor immunoreactive bands were seen at about 39, 37, and 33 kDa (Fig. 1A, lane 3), all of which were also shifted to about 33 kDa by the N-glycosidase treatment (Fig. 1A, lane 4). The band shifts by the deglycosylation indicate that all of the immunoreactive bands with different molecular weights are derived from identical protein molecules. N-glycosidase-insensitive immunoreactive bands were observed above 100 kDa in the lanes of the COS-7 cells (Fig. 1A, lanes 3 and 4), which probably reflected oligomerization of the receptor protein molecules as reported in adrenaline β2 and dopamine D3 receptors (Hebert et al., 1996; Nimchinsky et al., 1997). All of the immunoreactivity described here was absorbed completely by preincubation of the primary antibody with an excess amount of the fusion protein used as the immunogen (Fig. 1A, lane 2′). These results further strongly supported the antibody's specificity to rat EP3R.
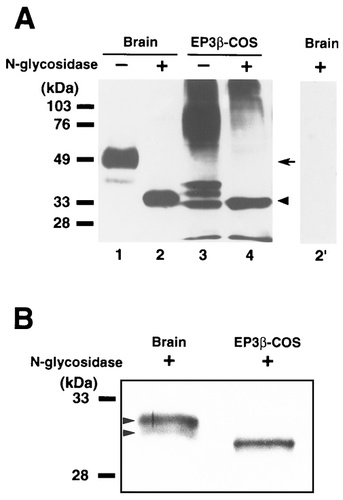
Characterization of the prostaglandin EP3 receptor (EP3R) protein in the rat brain and EP3R-expressing COS-7 cells by deglycosylation and immunoblotting. A: Protein immunopurified from rat brains (24 ng/lane) was incubated in a buffer without (lane 1) or with (lane 2) N-glycosidase. Membrane protein of rat EP3β receptor-expressing COS-7 cells (13 μg/lane) also was (lane 4) or was not (lane 3) subjected to deglycosylation. No immunoreactivity was detected after preincubation of the anti-EP3R antibody used in immunoblotting with an excess amount of the antigen (lane 2′). An arrow indicates the major immunoreactive bands in lane 1. An arrowhead indicates the apparently single band in lane 2. Immunoreactive bands seen at the bottom of lanes 3 and 4 indicate the front lines of the electrophoresis. B: After the deglycosylation, the protein immunopurified from rat brains (left) or the membrane fractions of the EP3β receptor-expressing COS-7 cells (right) was analyzed with higher resolution by electrophoresis in a urea-containing polyacrylamide gel. Arrowheads indicate the two adjacent immunoreactive bands.
In Figure 1B, the deglycosylated proteins were electrophoresed in a urea-containing polyacrylamide gel to analyze the electrophoretic mobilities with higher resolution. In the lane of the rat brain (Fig. 1B, left), we detected two adjacent immunoreactive bands at around 31 kDa (Fig. 1B, arrowheads), whereas only a single band was observed at 30 kDa in the lane of the COS-7 cells expressing rat EP3β receptor (Fig. 1B, right).
Distribution of EP3R in the nervous system
EP3R-like immunoreactivity (EP3R-LI) was distributed widely in the central and peripheral nervous systems, although the olfactory bulb and cerebellum did not show any immunoreactivity. EP3R-LI was observed mainly in neurons. However, intense immunoreactivity also was localized in the ependymal cells surrounding the lateral ventricle, the third and fourth ventricles, and the aqueduct (Figs. 2A, 4A–O, 8C, arrows). Other glial cells and nonneuronal cells of the choroid plexus, meninges, and blood vessels were not immunostained at all. In control immunostaining experiments, preincubation of the primary antibody with an excess amount of the immunogen abolished EP3R-LI (Fig. 2B) in all areas of the nervous system, demonstrating the high specificity of the immunostaining.
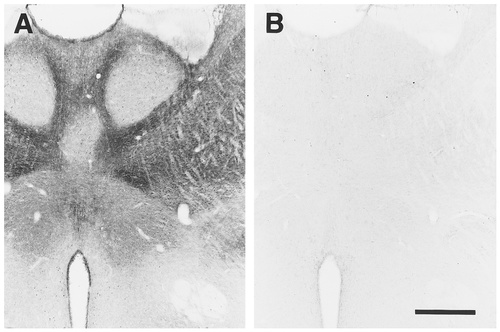
Preabsorption experiment to assess the specificity of immunoperoxidase staining with the anti-EP3R antibody. A: Intense immunoreactivity was detected in the anterior thalamic nuclear group by using the anti-EP3R antibody. B: Signals were not observed at all when the primary antibody was preincubated with an excess amount of the immunogen. Note that the immunoreactivity that was observed not only in the brain parenchyma but also in the ependyma was absorbed clearly. Scale bar = 500 μm.
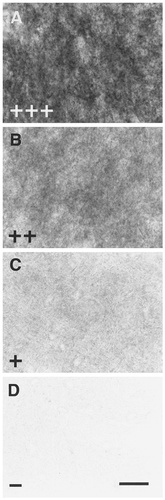
Internal standards for EP3R-like immunoreactivity (-LI). Intensity of EP3R-LI was ranked as follows: EP3R-LI was intense (+++) in neuropil of the paraventricular thalamic nucleus (A), moderate (++) in neuropil of the zonal and superficial gray layers of the superior colliculus (B), weak (+) in neuropil of the dorsal lateral geniculate nucleus (C), and negative (−) in neuropil of the paratenial thalamic nucleus (D). Scale bar = 20 μm.
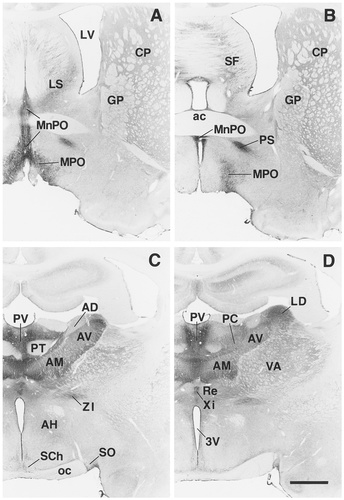
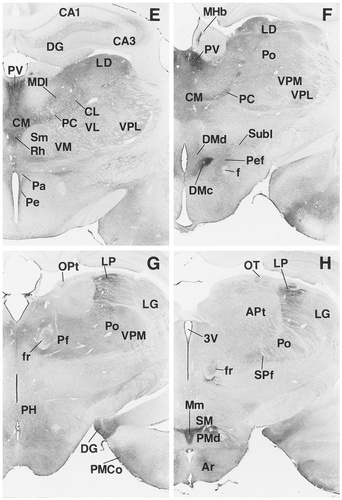
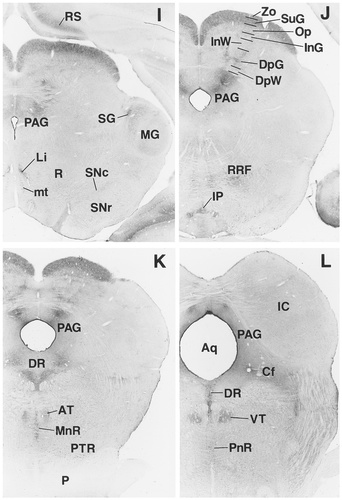
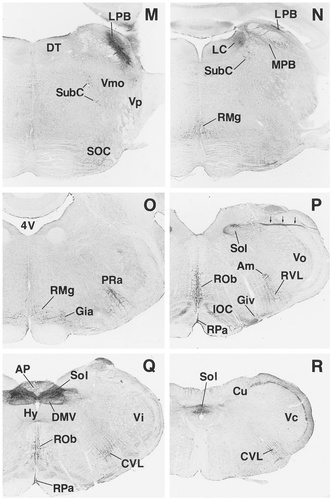
A–R: Overview of EP3R-LI from the preoptic and septal regions to the medulla oblongata. Arrows indicate the fibers of the vagus nerve that exhibit EP3R-LI. For a detailed explanation, see text. For abbreviations, see list. Scale bar = 1 mm.
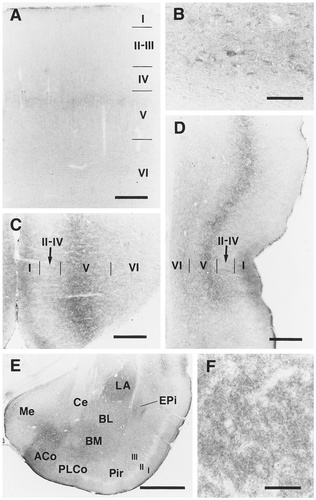
EP3R-LI in the cerebral cortex and amygdala. A: Primary somatosensory cortex. B: Neuronal cell bodies with EP3R-LI in layer VI of the primary somatosensory cortex. C: Cingulate cortex. D: Perirhinal and ectorhinal cortices. E: Amygdaloid body, piriform cortex, and endopiriform nucleus. F: Immunostained neuropil in the lateral amygdaloid nucleus. For abbreviations, see list. Scale bars = 300 μm in A,C,D; 50 μm in B,F; 1 mm in E.
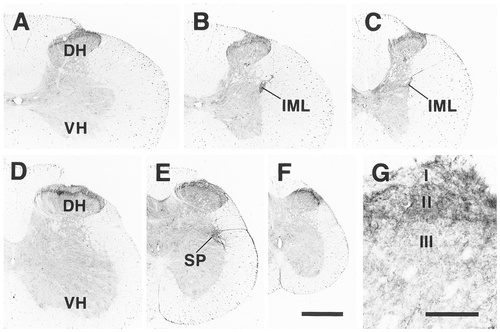
Distribution of EP3R-LI in the spinal cord. A: Middle cervical segment. B: Lower cervical segment. C: Middle thoracic segment. D: Middle lumbar segment. E: Upper sacral segment. F: Lower sacral segment. G: Immunostained neuropil in superficial laminae of the dorsal horn in a cervical segment. Signals seen in the white matter were not absorbed by preincubation of the primary antibody with an excess amount of the antigen. For abbreviations, see list. Scale bars = 500 μm in F (also applies to A–E); 100 μm in G.
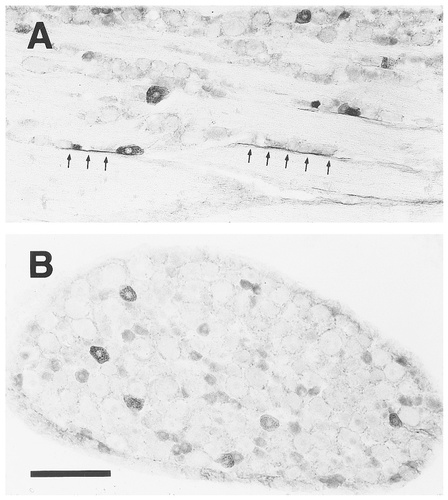
EP3R-LI in the trigeminal ganglion (A) and the dorsal root ganglion (B). Arrows indicate the axons that show EP3R-LI. Scale bar = 200 μm.
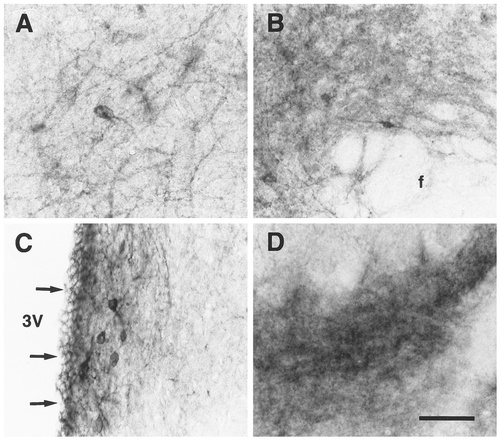
EP3R-LI in the intermediate part of the lateral septal nucleus (A), the perifornical nucleus (B), the periventricular hypothalamic nucleus (C), and the dorsal part of the premammillary nucleus (D). Arrows indicate the ependymal layer exhibiting EP3R-LI. 3V, third ventricle; f, fornix. Scale bar = 50 μm.
EP3R-LI was seen rather diffusely in neuropil of most regions of the brain and spinal cord by immunoperoxidase staining with the antibody (Fig. 3A–C), although neuronal cell bodies with EP3R-LI were observed clearly in some regions. Internal standards were employed to evaluate the intensity of EP3R-LI semiquantitatively: The immunoreactivity in neuropil was intense (+++) in the paraventricular thalamic nucleus (Fig. 3A), moderate (++) in the zonal and superficial gray layers of the superior colliculus (Fig. 3B), weak (+) in the dorsal lateral geniculate nucleus (Fig. 3C), and negative (−) in the paratenial thalamic nucleus (Fig. 3D). The distribution pattern of EP3R-LI is summarized in Table 1 in terms of the intensity of EP3R-LI in neuropil. Table 1 also lists the regions in which neuronal cell bodies with EP3R-LI were visible.
| Sites | Neuropil1 | Cell bodies2 |
|---|---|---|
| Olfactory bulb | − | − |
| Neocortex | ||
| Layers I–IV and V (deep) | − | − |
| Layer V (superficial) | + | − |
| Layer VI | − | + (scattered) |
| Prelimbic, infralimbic, cingulate, and retrosplenial cortices | ||
| Layer I | + | − |
| Layers II–IV and VI | − | − |
| Layer V | +∼++ | − |
| Insular, ectorhinal, and perirhinal cortices | ||
| Layers I and V | +∼++ | − |
| Layers II–IV | − | − |
| Layer VI | − | + (scattered) |
| Piriform cortex | ||
| Layers I and III | + | − |
| Layer II | − | − |
| Entorhinal cortex | ||
| Layers I–III | − | − |
| Layers IV–VI | +∼++ | − |
| Ammon's horn | ||
| Stratum oriens | − | + (scattered) |
| Pyramidal cell layer | − | + |
| Stratum radiatum | − | + (scattered) |
| Stratum lacunosum-moleculare | + | − |
| Dentate gyrus | ||
| Molecular layer | + | − |
| Granule cell layer | − | + |
| Polymorph layer | − | + (scattered) |
| Subiculum | + | − |
| Postsubiculum | + | − |
| Presubiculum | − | − |
| Parasubiculum | − | − |
| Septal region | ||
| Lateral septal nucleus | ||
| Dorsal part | − | − |
| Ventral part | + | + |
| Intermediate part | +∼++ | + |
| Medial septal nucleus | − | − |
| Septofimbrial nucleus | + | + |
| Nucleus of the diagonal band | − | − |
| Basal ganglia | ||
| Caudate-putamen | + | − |
| Accumbens nucleus | +∼++ | − |
| Globus pallidus | + | − |
| Islands of Calleja | + | − |
| Endopiriform nucleus | +∼++ | − |
| Entopeduncular nucleus | − | − |
| Claustrum | − | − |
| Amygdala | ||
| Anterior cortical amygdaloid nucleus | +∼++ | − |
| Posteromedial cortical amygdaloid nucleus | ||
| Rostral part | +∼++ | − |
| Caudal part | − | − |
| Posterolateral cortical amygdaloid nucleus | ||
| Rostral part | + | − |
| Caudal part | − | − |
| Lateral amygdaloid nucleus | +∼++ | − |
| Basolateral amygdaloid nucleus | + | − |
| Medial amygdaloid nucleus | + | − |
| Basomedial amygdaloid nucleus | +∼++ | − |
| Central amygdaloid nucleus | + | − |
| Anterior amygdaloid area | ± | − |
| Preoptic region and hypothalamus | ||
| Medial preoptic area | ++∼+++ | + |
| Lateral preoptic area | − | − |
| Median preoptic nucleus | +++ | + |
| Anteroventricular periventricular nucleus | − | − |
| Ventromedial preoptic nucleus | − | − |
| Parastrial nucleus | +++ | − |
| Suprachiasmatic nucleus | − | − |
| Supraoptic nucleus | − | − |
| Anterior hypothalamic area | ||
| Anterior part | + | − |
| Posterior part | + | + (scattered) |
| Periventricular nucleus | +∼++ | + |
| Paraventricular hypothalamic nucleus | +∼++ | − |
| Dorsal hypothalamic area | + | + (scattered) |
| Dorsomedial hypothalamic nucleus | ||
| Compact part | +++ | − |
| Dorsal and ventral parts | + | + (scattered) |
| Sites | Neuropil1 | Cell bodies2 |
| Perifornical nucleus | + | + (scattered) |
| Subincertal nucleus | + | + (scattered) |
| Posterior hypothalamic area | + | + (scattered) |
| Arcuate nucleus | ||
| Lateroposterior part | + | + |
| Other parts | − | − |
| Premammillary nucleus | ||
| Dorsal part | ++∼+++ | − |
| Ventral part | − | − |
| Supramammillary nucleus | ||
| Dorsal part | − | − |
| Ventral part | ++∼+++ | − |
| Medial mammillary nucleus | ||
| Median part | +++ | − |
| Medial and lateral parts | + | − |
| Thalamus | ||
| Anteroventral nucleus | ++∼+++ | − |
| Anteromedial nucleus | ++∼+++ | + |
| Anterodorsal nucleus | +∼++ | − |
| Mediodorsal nucleus | ||
| Lateral part | + | + |
| Medial and central parts | − | − |
| Ventral anterior nucleus | + | + |
| Ventral lateral nucleus | + | + |
| Ventral medial nucleus | + | + |
| Ventral posteromedial nucleus | + | − |
| Ventral posterolateral nucleus | + | − |
| Laterodorsal nucleus | ||
| Rostral part | +++ | + |
| Caudal part | ++ | + |
| Lateral posterior nucleus | ||
| Mediocaudal part | ++ | − |
| Other parts | +∼++ | + |
| Posterior nuclear group | + | + |
| Central lateral nucleus | +∼++ | + |
| Central medial nucleus | ++∼+++ | + |
| Paracentral nucleus | ++∼+++ | + |
| Parafascicular nucleus | + | + |
| Subparafascicular nucleus | + | + |
| Paraventricular nucleus | +++ | − |
| Intermediodorsal nucleus | +∼++ | + |
| Paratenial nucleus | − | − |
| Interanterodorsal nucleus | +++ | + |
| Interanteromedial nucleus | +++ | + |
| Rhomboid nucleus | ||
| Rostral part | − | − |
| Caudal part | ++∼+++ | + |
| Reuniens nucleus | + | + |
| Xiphoid nucleus | ++∼+++ | + |
| Submedius (or gelatinosus) nucleus | − | − |
| Dorsal lateral geniculate nucleus | + | − |
| Ventral lateral geniculate nucleus | +∼++ | − |
| Suprageniculate nucleus | + | + |
| Medial geniculate nucleus | + | + |
| Habenula | ||
| Medial habenular nucleus | ||
| Lateral part | +++ | + (scattered) |
| Medial part | − | − |
| Lateral habenular nucleus | − | − |
| Zona incerta | ||
| Rostral part | ++ | − |
| Intermediate and caudal parts | − | − |
| Pretectum | ||
| Nucleus of the optic tract | + | − |
| Anterior pretectal nucleus | − | − |
| Medial pretectal nucleus | − | − |
| Olivary pretectal nucleus | + | − |
| Posterior pretectal nucleus | − | − |
| Precommissural nucleus | − | − |
| Superior colliculus | ||
| Zonal layer | ++ | − |
| Superficial gray layer | ++ | − |
| Optic nerve layer | + | − |
| Intermediate gray layer | − | − |
| Intermediate white layer | − | − |
| Deep gray layer | +∼++ | − |
| Deep white layer | − | − |
| Periaqueductal gray | ||
| Dorsolateral part | ||
| Anterior one-third | + | + |
| Posterior two-thirds | ++ | − |
| Other parts | ||
| Rostral portion | − | − |
| Caudal portion | + | − |
| Edinger-Westphal nucleus | − | − |
| Oculomotor nucleus | − | − |
| Sites | Neuropil1 | Cell bodies2 |
| Trochlear nucleus | − | − |
| Terminal nuclei of the accessory optic tract | − | − |
| Retrorubral field | +∼++ | − |
| Ventral tegmental area | − | − |
| Substantia nigra | − | − |
| Red nucleus | − | − |
| Inferior colliculus | − | − |
| Cuneiform nucleus | +∼++ | − |
| Mesencephalic trigeminal nucleus | − | − |
| Rostral linear nucleus of the raphe | + | + (scattered) |
| Caudal linear nucleus of the raphe | − | − |
| Dorsal raphe nucleus | ++ | + |
| Median raphe nucleus | +∼++ | + |
| Anterior tegmental nucleus | + | − |
| Ventral tegmental nucleus | + | − |
| Interpeduncular nucleus | ||
| Dorsomedial and dorsolateral subnuclei | +∼++ | − |
| Other subnuclei | − | − |
| Lateral parabrachial nucleus | ||
| External, central, and crescent parts | ++∼+++ | − |
| Dorsal part | ++ | − |
| Ventral and internal parts | − | − |
| Medial parabrachial nucleus | ||
| Rostral part | − | − |
| Caudal part | + | + |
| Locus coeruleus | ++ | + (scattered) |
| Subcoeruleus nucleus | − | + (scattered) |
| Pontine raphe nucleus | + | + |
| Pontine tegmental reticular nucleus | − | + (scattered) |
| Dorsal tegmental nucleus | − | − |
| Superior olivary complex | − | − |
| Nucleus of the trapezoid body | − | − |
| Motor trigeminal nucleus | − | − |
| Principal sensory trigeminal nucleus | − | − |
| Pontine nuclei | − | − |
| Cerebellum | − | − |
| Nucleus of the solitary tract | ||
| Commissural part | +++ | − |
| Medial part | ++∼+++ | − |
| Intermediate part | ++∼+++ | ± |
| Ventrolateral part | + | − |
| Dorsal motor nucleus of the vagus nerve | ++ | + (scattered) |
| Sites | Neuropil1 | Cell bodies2 |
| Raphe magnus nucleus | ++ | + |
| Raphe obscurus nucleus | ++ | + |
| Raphe pallidus nucleus | ++ | + |
| Gigantocellular reticular nucleus | ||
| Alpha and ventral parts | ||
| Lateral portion | ++ | + |
| Medial and intermediate portions | − | − |
| Other parts | − | − |
| Area postrema | ± | − |
| Cochlear nuclei | − | − |
| Abducens nucleus | − | − |
| Facial nucleus | − | − |
| Vestibular nuclei | − | − |
| Hypoglossal nucleus | − | − |
| Ambiguus nucleus | ||
| Rostral part | ++ | − |
| Caudal part | − | − |
| External cuneate nucleus | − | − |
| Cuneate nucleus | − | − |
| Gracile nucleus | − | − |
| Inferior olivary complex | ||
| Principal nucleus | + | − |
| Other nuclei | − | − |
| Prepositus hypoglossal nucleus | − | − |
| Lateral reticular nucleus | − | − |
| Rostroventrolateral reticular nucleus | + | + (scattered) |
| Caudoventrolateral reticular nucleus | + | + (scattered) |
| Spinal trigeminal nucleus | ||
| Caudal part | ||
| Lamina I | + | − |
| Lamina II | ++∼+++ | − |
| Other laminae | − | − |
| Other parts | − | − |
| Spinal cord | ||
| Lamina I | + | − |
| Lamina II | ++∼+++ | − |
| Other laminae | − | − |
| Intermediolateral nucleus | ++ | + (scattered) |
| Sacral parasympathetic nucleus | ++ | + |
| Trigeminal ganglion | + | + |
| Dorsal root ganglion | + | + |
| Nodose ganglion | + | + |
| Superior cervical ganglion | + | + |
| Stellate ganglion | + | + |
- 1 The intensity of prostaglandin EP3 receptor immunoreactivity in neuropil was scored visually by using the internal standard (see Fig. 3): 111, intense; 11, moderate; 1, weak; 2, negative.
- 2 The regions where immunostained neuronal cell bodies were or were not found are shown by 1 and 2, respectively. The regions where scattered immunopositive neuronal cell bodies were observed are denoted by (scattered).
Cerebral cortex.
Layer V showed EP3R-LI in neuropil: The immunoreactivity was moderate to weak in the prelimbic, infralimbic, cingulate, retrosplenial, insular, ectorhinal, and perirhinal cortices (Figs. 4I, 5C,D) and weak in the superficial zone of layer V of the neocortex (Fig. 5A). However, the deep zone of layer V of the neocortex showed no EP3R-LI. Moderate or weak EP3R-LI was observed in the neuropil of layer I of the insular, ectorhinal, and perirhinal cortices (Fig. 5D), and weak immunoreactivity was detected in the neuropil of layer I of the prelimbic, infralimbic, cingulate, and retrosplenial cortices (Fig. 5C). Neuronal cell bodies exhibiting weak EP3R-LI were scattered in layer VI of the neocortex, the insular cortex, the ectorhinal cortex, and the perirhinal cortex (Fig. 5B), although no cell bodies with EP3R-LI were seen in the prelimbic, infralimbic, cingulate, or retrosplenial cortices. EP3R-LI was moderate to weak in the neuropil of layers IV–VI of the entorhinal cortex and weak in the neuropil of layers I and III of the piriform cortex (Fig. 5E). However, EP3R-LI was not detected in layers I–III of the entorhinal cortex or in layer II of the piriform cortex. Cell bodies with immunoreactivity were not observed in the entorhinal or piriform cortices.
Hippocampus.
Weak EP3R-LI was seen in neuronal cell bodies of Ammon's horn (Fig. 4C–E). Many pyramidal neurons exhibited immunoreactivity, and a few neuronal cells with EP3R-LI were scattered in the stratum oriens and stratum radiatum. Neuropil was weakly immunostained in the stratum lacunosum-moleculare. In the dentate gyrus (Fig. 4E,G,H), weak EP3R-LI was observed in many neuronal cells of the granule cell layer and in some neuronal cells dispersed in the polymorph layer. The molecular layer exhibited weak EP3R-LI in the neuropil. EP3R-LI also was weak in the neuropil of the subiculum and postsubiculum, but no immunoreactivity was detected in the presubiculum or parasubiculum.
Amygdala.
EP3R-LI in neuropil was moderate to weak in the anterior cortical amygdaloid nucleus and in the rostral part of the posteromedial cortical amygdaloid nucleus, and it was weak in the rostral part of the posterolateral cortical amygdaloid nucleus (Figs. 4F–H, 5E). However, no EP3R-LI was detected in the caudal parts of the posteromedial and posterolateral cortical amygdaloid nuclei. Moderate or weak EP3R-LI was observed in the neuropil of the lateral and basomedial amygdaloid nuclei (Fig. 5E,F). Weak EP3R-LI was seen in the neuropil of the basolateral, medial, and central amygdaloid nuclei (Fig. 5E). EP3R-LI in the anterior amygdaloid area was weak or negative. No cell bodies with EP3R-LI could be detected throughout the amygdaloid body.
Basal ganglia and septal and basal forebrain regions.
Moderate or weak EP3R-LI was detected in the neuropil of the endopiriform nucleus (Fig. 5E), although the claustrum showed no immunoreactivity. EP3R-LI was moderate to weak in the neuropil of the accumbens nucleus and was weak in the neuropil of the caudate-putamen, globus pallidus, and islands of Calleja (Fig. 4A,B). Immunoreactivity was not observed in the entopeduncular nucleus. No neuronal cell bodies with EP3R-LI were observed in the basal ganglia or in the basal forebrain region.
In the lateral septal nucleus (Fig. 4A), neuronal cell bodies and fine processes with moderate or weak EP3R-LI were distributed sparsely in the intermediate and ventral parts (Fig. 8A), whereas no immunoreactivity was seen in the dorsal part. The septofimbrial nucleus also contained a few neuronal cell bodies with weak EP3R-LI (Fig. 4B). However, no EP3R-LI was detected in the medial septal nucleus or in the nucleus of the diagonal band.
Preoptic region and hypothalamus.
Many neuronal cell bodies and fine processes with intense EP3R-LI were densely localized in the median preoptic nucleus and the medial preoptic area (Fig. 4A,B), as shown previously by Nakamura et al. (1999). The parastrial nucleus also contained a number of fine processes with intense EP3R-LI, but immunostained neuronal cell bodies were not detected in the nucleus. No EP3R-LI was observed in the lateral preoptic area, anteroventricular periventricular nucleus, or ventromedial preoptic nucleus.
EP3R-LI was intense in the neuropil of the compact part of the dorsomedial hypothalamic nucleus, as reported previously (Nakamura et al., 1999), and weak in the neuropil of the dorsal and ventral parts of the nucleus (Fig. 4F), although only the dorsal and ventral parts contained a few neuronal cell bodies with weak EP3R-LI. The immunostained neuropil of the dorsal and ventral parts of the dorsomedial hypothalamic nucleus seemed to spread over the perifornical and subincertal nuclei (Fig. 4F), in which scattered neuronal cell bodies exhibited moderate or weak EP3R-LI (Fig. 8B). Both of the periventricular and paraventricular hypothalamic nuclei showed moderate or weak EP3R-LI in the neuropil (Fig. 4D,E), whereas only the periventricular nucleus contained neuronal cell bodies with intense immunoreactivity (Fig. 8C). Weak EP3R-LI was observed in the neuropil of the anterior hypothalamic area (Fig. 4C), whereas neuronal cell bodies with moderate or weak EP3R-LI were scattered only in the posterior part of the area. The dorsal and posterior hypothalamic areas contained moderately or weakly immunostained neuronal cell bodies that were dispersed in the neuropil with weak EP3R-LI (Fig. 4G). However, EP3R-LI was not detected in the suprachiasmatic nucleus or the supraoptic nucleus.
In the mammillary body (Fig. 4H), intense or moderate EP3R-LI was observed in the neuropil of a region comprised of the median part of the medial mammillary nucleus, the dorsal part of the premammillary nucleus, and the ventral part of the supramammillary nucleus (Fig. 8D). Weak EP3R-LI was seen in the neuropil of the medial and lateral parts of the medial mammillary nucleus. However, no cell bodies showing EP3R-LI were distributed in those mammillary nuclei. Neuronal cell bodies with moderate or weak EP3R-LI were detected in the lateroposterior part of the arcuate nucleus, in which weak immunoreactivity also was detected in the neuropil.
Thalamus, epithalamus, and subthalamus.
In the anterior nuclear group (Fig. 4C,D), EP3R-LI was intense or moderate in the neuropil of the anteroventral and anteromedial nuclei and moderate or weak in the neuropil of the anterodorsal nucleus. Granular EP3R-LI was detected in many neuronal cell bodies in the anteromedial nucleus (Fig. 9A), whereas no cell bodies with EP3R-LI were seen in the anteroventral or anterodorsal nuclei (Fig. 9B). In the mediodorsal nucleus (Fig. 4E), EP3R-LI was detected only in the lateral part, in which neuronal cell bodies with granular EP3R-LI were observed, and the neuropil also was immunostained weakly. The ventral nuclear complex (Fig. 4D–G) exhibited weak EP3R-LI in the neuropil, and neuronal cell bodies with weak EP3R-LI were distributed in the ventral anterior, ventral lateral, and ventral medial nuclei (Fig. 9C). However, no cell bodies with EP3R-LI were detected in the ventral posteromedial nucleus or the ventral posterolateral nucleus.
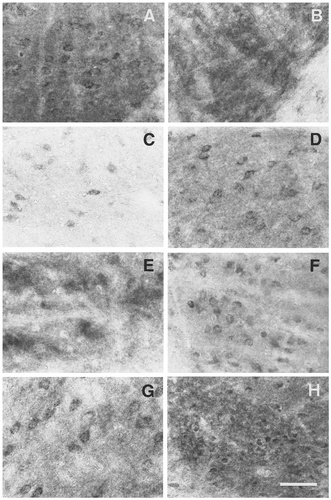
EP3R-LI in the thalamus. A: Anteromedial nucleus. B: Anteroventral nucleus. C: Ventral medial nucleus. D: Laterodorsal nucleus. E: Mediocaudal part of the lateral posterior nucleus. F: Mediorostral part of the lateral posterior nucleus. G: Central lateral nucleus. H: Reuniens nucleus. Scale bar = 50 μm.
In the lateral nuclear group, the laterodorsal nucleus showed intense EP3R-LI in the neuropil of the rostral part (Fig. 4D) and moderate EP3R-LI in the neuropil of the caudal part (Fig. 4E,F), and many neuronal cell bodies with granular EP3R-LI were distributed throughout the rostrocaudal axis of the laterodorsal nucleus (Fig. 9D). The lateral posterior nucleus exhibited moderate or weak EP3R-LI in the neuropil (Fig. 4G,H). Although no cell bodies with EP3R-LI were observed in the mediocaudal part of the lateral posterior nucleus (Fig. 9E), moderate or weak immunoreactivity was detected in neuronal cell bodies in the other parts (Fig. 9F). The posterior nuclear group (Fig. 4F–H) contained many neuronal cell bodies with moderate or weak EP3R-LI and exhibited weak immunoreactivity in the neuropil.
Many neuronal cell bodies showing moderate or weak EP3R-LI were distributed throughout the intralaminar nuclear group (Fig. 9G). EP3R-LI was intense to moderate in the neuropil of the central medial and paracentral nuclei, moderate to weak in the neuropil of the central lateral nucleus (Fig. 4E,F), and weak in the neuropil of the parafascicular and subparafascicular nuclei (Fig. 4G,H). In the midline nuclear group (Fig. 4C–F), EP3R-LI was intense in the neuropil of the paraventricular, interanterodorsal, and interanteromedial nuclei and was moderate or weak in the neuropil of the intermediodorsal nucleus. Many neuronal cell bodies showing moderate EP3R-LI were distributed in the interanterodorsal, interanteromedial, and intermediodorsal nuclei, although the paraventricular nucleus contained no cell bodies with EP3R-LI (Fig. 3A). Intense or moderate EP3R-LI was observed in the neuropil of the xiphoid nucleus and of the caudal part of the rhomboid nucleus, and weak EP3R-LI was observed in the neuropil of the reuniens nucleus. Neuronal cell bodies showing intense or moderate EP3R-LI were densely packed in the caudal part of the rhomboid nucleus (Fig. 9H), and a few neuronal cell bodies with weak EP3R-LI were distributed in the reuniens and xiphoid nuclei. No EP3R-LI was detected in the paratenial nucleus (Fig. 3D), the rostral part of the rhomboid nucleus, or the submedius nucleus.
All parts of the lateral geniculate nucleus (Fig. 4G,H) showed weak-to-moderate immunoreactivity in the neuropil but did not contain any cell bodies with EP3R-LI (Fig. 3C). Neuronal cell bodies with weak or moderate EP3R-LI were localized in the suprageniculate and medial geniculate nuclei, which also exhibited weak EP3R-LI in the neuropil (Fig. 4I).
In the lateral part of the medial habenular nucleus, the neuropil and a few neuronal cell bodies with intense EP3R-LI were packed densely (Figs. 4F, 10A). However, no immunoreactivity was seen in the lateral habenular nucleus or in the medial part of the medial habenular nucleus. We detected weak EP3R-LI on the fibers of the fasciculus retroflexus (Fig. 4G,H) and also observed several neuronal cell bodies and fine dendritic processes exhibiting intense EP3R-LI by the side of the fasciculus retroflexus (Fig. 10B). In the zona incerta, the neuropil of the rostral part was immunostained moderately (Fig. 4C,D), but no immunoreactivity was detected in the intermediate or caudal parts.
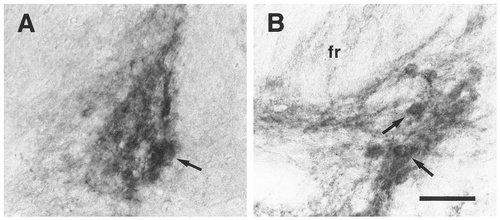
EP3R-LI in the medial habenular nucleus (A) and the fasciculus retroflexus (B). Neuronal cell bodies with intense EP3R-LI (A, arrow) were distributed sparsely in the lateral part of the medial habenular nucleus. Arrows in B indicate the neuronal cells exhibiting EP3R-LI located by the side of the fasciculus retroflexus (fr). Scale bar = 50 μm.
Midbrain.
Among the raphe nuclei belonging to the midbrain, the dorsal raphe nucleus contained many neuronal cell bodies and fine dendritic processes with moderate EP3R-LI (Figs. 4K,L, 13C). The median raphe nucleus also exhibited moderate or weak EP3R-LI in neuronal cells and a few fine processes (Figs. 4K, 13D). The rostral linear nucleus of the raphe contained a small number of intensely immunostained neuronal cell bodies and fine dendritic processes (Fig. 4I), whereas no EP3R-LI was seen in the caudal linear nucleus of the raphe.
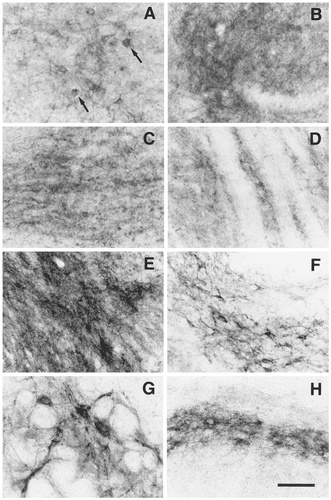
EP3R-LI in the anterior (A) and posterior (B) portions of the periaqueductal gray, the interpeduncular nucleus (C), the cuneiform nucleus (D), the lateral parabrachial nucleus (E), the medial parabrachial nucleus (F), the alpha part of the parvicellular reticular nucleus (G), and the superficial laminae of the caudal part of the spinal trigeminal nucleus (H). In the periaqueductal gray, neuronal cells with EP3R-LI (A, arrows) were observed in the anterior part, whereas the posterior part showed EP3R-LI only in neuropil (B). Scale bar = 50 μm.

EP3R-LI in the vagal pathway. In the nucleus of the solitary tract, EP3R-LI was observed in the commissural (A), medial (B), and intermediate (C) parts. EP3R-LI also was seen in the solitary tract (D) and the nodose ganglion (E). Scale bar = 50 μm.
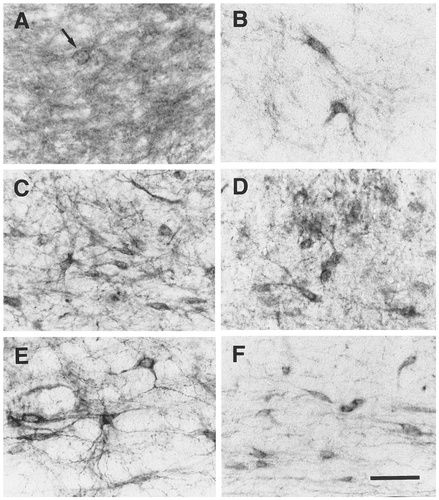
EP3R-LI in the monoamine-containing nuclei. A: Locus coeruleus. Neuronal cell bodies with EP3R-LI (arrow) were distributed sparsely. B: Subcoeruleus nucleus. C: Dorsal raphe nucleus. D: Median raphe nucleus. E: Raphe magnus nucleus. F: Pontine tegmental reticular nucleus. Scale bar = 50 μm.
In the dorsolateral part of the periaqueductal gray, the neuropil was immunostained moderately in the posterior two-thirds (Fig. 4J–L) and weakly in the anterior one-third (Fig. 4I). Several neuronal cell bodies with weak EP3R-LI were detected only in the anterior one-third of the dorsolateral part (Fig. 11A, arrows), but not in the posterior two-thirds (Fig. 11B). The other parts of the periaqueductal gray showed weak EP3R-LI in the neuropil of the caudal portion (Fig. 4K,L), although no immunoreactivity was detected in the rostral portion of the other parts of the periaqueductal gray.
EP3R-LI in the superior colliculus (Fig. 4I–K) was moderate in the neuropil of the zonal and superficial gray layers (Fig. 3B), moderate to weak in the neuropil of the deep gray layer, and weak in the neuropil of the optic nerve layer; whereas EP3R-LI was not seen in the intermediate gray layer, the intermediate white layer, or the deep white layer. In the pretectal area (Fig. 4G,H), EP3R-LI was weak in the neuropil of the olivary pretectal nucleus and the nucleus of the optic tract, although no immunoreactivity was seen in the anterior pretectal nucleus, medial pretectal nucleus, posterior pretectal nucleus, or precommissural nucleus. No neuronal cell bodies with EP3R-LI were found in the pretectal area or the superior colliculus.
Moderate or weak EP3R-LI was detected in the neuropil of the dorsomedial and dorsolateral subnuclei of the interpeduncular nucleus (Figs. 4J, 11C), whereas no immunoreactivity was seen in the other subnuclei of the nucleus. The retrorubral field (Fig. 4J) and the cuneiform nucleus (Figs. 4L, 11D) also showed moderate or weak EP3R-LI in the neuropil, and the anterior and ventral tegmental nuclei exhibited weak immunoreactivity in the neuropil (Fig. 4K,L). However, neuronal cell bodies with EP3R-LI were not seen in the interpeduncular nucleus, cuneiform nucleus, retrorubral field, or anterior or ventral tegmental nuclei. On the mammillotegmental tract, we could also observe weak EP3R-LI (Fig. 4I). No EP3R-LI was detected in the Edinger-Westphal nucleus, oculomotor or trochlear nuclei, terminal nuclei of the accessory optic tract, substantia nigra, ventral tegmental area, red nucleus, inferior colliculus, or mesencephalic trigeminal nucleus.
Pons.
In the lateral parabrachial nucleus (Fig. 4M,N), EP3R-LI was intense to moderate in the neuropil of the external, central, and crescent parts and moderate in the neuropil of the dorsal part, although no EP3R-LI was shown in the ventral or internal parts. Cell bodies with EP3R-LI could not be detected in the lateral parabrachial nucleus (Fig. 11E). Some neuronal groups comprised of small cells exhibited intense EP3R-LI in the caudal part of the medial parabrachial nucleus (Figs. 4N, 11F), in which weak EP3R-LI also was observed in the neuropil, whereas no immunoreactivity was seen in the rostral part of the medial parabrachial nucleus.
Many dendritic processes were immunostained moderately in the locus coeruleus (Fig. 4N), although the numbers of neuronal cell bodies exhibiting EP3R-LI were small (Fig. 13A, arrow). In the subcoeruleus nucleus, we detected scattered neuronal cell bodies showing intense EP3R-LI (Figs. 4M, 13B). This positive cell group in the subcoeruleus nucleus extended in a dorsocaudal direction and finally fused with the locus coeruleus (Fig. 4N).
Neuronal cell bodies exhibiting intense EP3R-LI were scattered in the pontine tegmental reticular nucleus (Figs. 4K, 13F). In the pontine raphe nucleus, some neuronal cell bodies showed moderate or weak EP3R-LI, and weak immunoreactivity also was detected in the neuropil (Fig. 4L). We observed scattered neuronal cell bodies and fine processes showing intense EP3R-LI in the ventral portion of the alpha part of the parvicellular reticular nucleus (Figs. 4O, 11G). No EP3R-LI was seen in the dorsal tegmental nucleus, superior olivary complex, nucleus of the trapezoid body, motor trigeminal nucleus, principal sensory trigeminal nucleus, or pontine nuclei.
Medulla oblongata.
In the nucleus of the solitary tract (Fig. 4P–R), EP3R-LI was intense in the neuropil of the commissural part (Fig. 12A), intense to moderate in the neuropil of the medial and intermediate parts, and weak in the neuropil of the ventrolateral part. In the rostral half of the nucleus, the medial (Fig. 12B) and intermediate parts (Fig. 12C) contained nerve fibers with intense or moderate EP3R-LI. The intermediate part also possessed intense and granular immunosignals forming a soma-like shape (Fig. 12C). We could not judge whether the immunoreactive structures with the soma-like shape were neuronal cell bodies with EP3R-LI, because it was possible that axon terminals with EP3R-LI surrounded immunonegative neuronal cell bodies. Intense EP3R-LI also was observed in many axons of the vagus nerve (Fig. 4P, arrows; Fig. 12D).
In the dorsal motor nucleus of the vagus nerve (Fig. 4Q), moderate EP3R-LI was seen in the neuropil, and several neuronal cell bodies showing weak EP3R-LI were detected especially in the medial portion (Fig. 14A). The ambiguus nucleus exhibited moderate EP3R-LI in the neuropil of the rostral part (Figs. 4P, 14B), although the caudal part showed no immunoreactivity. In an area immediately ventral to the ambiguus nucleus, which belongs to the rostroventrolateral or caudoventrolateral reticular nucleus and was referred to as the external formation of the ambiguus nucleus in a previous study (Bieger and Hopkins, 1987), we observed scattered neuronal cell bodies and fine processes with intense or moderate EP3R-LI (Fig. 4P–R; Fig. 14B, arrows). Because signals in the area postrema had barely disappeared by preincubation of the primary antibody with the immunogen, we could not evaluate EP3R-LI in the region.
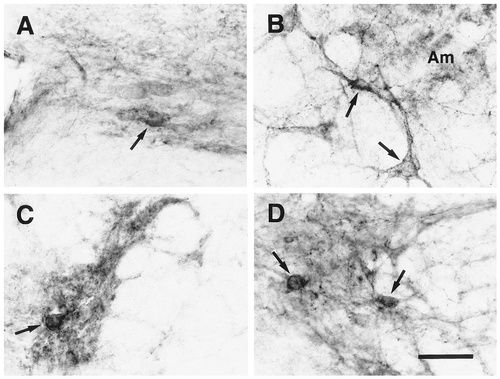
EP3R-LI in the central autonomic nervous system. A: Dorsal motor nucleus of the vagus nerve. B: Neuronal cells exhibiting EP3R-LI (arrows) located immediately ventral to the ambiguus nucleus (Am). C: Intermediolateral nucleus. D: Sacral parasympathetic nucleus. Arrows indicate the neuronal cell bodies that exhibit EP3R-LI. Scale bar = 50 μm.
Neuronal cell bodies and fine dendritic processes exhibiting intense EP3R-LI were distributed in the raphe magnus, raphe obscurus, and raphe pallidus nuclei (Figs. 4N–Q, 13E). The positive neuronal group in these raphe nuclei extended into the lateral portions of the alpha and ventral parts of the gigantocellular reticular nucleus (Fig. 4O,P). In the caudal part of the spinal trigeminal nucleus, only laminae I and II exhibited EP3R-LI in the neuropil (Fig. 4R). The EP3R-LI was intense to moderate in lamina II and weak in lamina I (Fig. 11H). Weak EP3R-LI was detected in the neuropil of the medial part of the principal nucleus of the inferior olivary complex (Fig. 4P), although other parts of the inferior olivary complex did not exhibit EP3R-LI. No EP3R-LI was seen in the cochlear nuclei, abducens nucleus, facial nucleus, vestibular nuclei, hypoglossal nucleus, external cuneate and cuneate nuclei, gracile nucleus, prepositus hypoglossal nucleus, or lateral reticular nucleus.
Spinal cord and peripheral ganglia.
In all of the spinal segments (Fig. 6A–F), the neuropil of lamina II and lamina I showed moderate and weak EP3R-LI, respectively (Fig. 6G). In some sequential sections from each spinal segment, intense EP3R-LI was detected in the neuropil of the inner zone of lamina II. Neither laminae contained any neuronal cell bodies with EP3R-LI. The intermediolateral nucleus of the cervicothoracic cord showed moderate EP3R-LI in the neuropil and contained a few neuronal cell bodies with weak immunoreactivity (Figs. 6B,C, 14C). Several neuronal cell bodies and fine processes exhibited moderate EP3R-LI in the sacral parasympathetic nucleus (Figs. 6E, 14D).
In the trigeminal and dorsal root ganglia (Fig. 7), many neuronal cell bodies showed EP3R-LI. Most of the positive cells exhibited moderate or weak EP3R-LI and appeared to be classified as small neurons, whereas a small number of large or medium-sized positive cells showed intense immunoreactivity. The nodose ganglion also contained many neuronal cell bodies with intense or moderate EP3R-LI (Fig. 12E). In those sensory ganglia, EP3R-LI was found on the surface membrane of the neuronal cell bodies, and granular immunosignals were observed frequently in the cytoplasm. Axons with EP3R-LI also were detected in those ganglia, and some were continuous with immunostained cell bodies (Fig. 7A, arrows). In the sympathetic ganglia, a small number of the superior cervical and stellate ganglion cells exhibited EP3R-LI (data not shown).
DISCUSSION
Glycosylated EP3R protein
In the immunoblotting analysis, the antibody specific to rat EP3R exhibited several immunoreactive bands with different molecular weights in immunopurified protein from rat brains and in membrane protein of rat EP3β receptor-expressing COS-7 cells. After deglycosylation, most of those immunoreactive bands came to show an apparently single entity with a molecular weight of ≈ 33 kDa. This result indicates that EP3R protein molecules are modified posttranslationally with different sizes of carbohydrate moieties, and, thereby, several forms of glycoproteins are produced. In a recent study, mutations at two deduced N-glycosylation sites of mouse EP3α receptor affected the affinity and specificity of the ligand binding (Huang and Tai, 1998). Therefore, the various types of carbohydrate moieties may have some modulatory roles in the physiologic functions of EP3R.
To obtain further detailed information on EP3R protein, the deglycosylated receptor protein was analyzed with electrophoresis in a urea-containing polyacrylamide gel, which could well separate proteins at around 30 kDa. Two adjacent immunoreactive bands were detected in the lane of the brain, whereas only a single band was seen in the lane of the COS-7 cells. Because rat EP3R has three isoforms with similar molecular weights deduced from their amino-acid sequences, 39.9 kDa (EP3α), 39.6 kDa (EP3β), and 39.5 kDa (EP3γ; Takeuchi et al., 1993, Takeuchi et al., 1994; Kitanaka et al., 1996), it is likely that the two bands were derived from the isoforms endogenously expressed in the rat brain. On the other hand, the single band may be formed by EP3β isoform expressed in the culture cells. Moreover, the two immunoreactive bands in the brain showed higher molecular weights than the single band in the COS-7 cells. This may arise from protein modifications specific in the brain, such as the addition of phosphate groups on the protein, as seen in other receptors (Ji et al., 1998).
Correlations between EP3R distribution and functions
The distribution of EP3R-LI observed in this study was highly similar to that of PGE2 binding sites identified by quantitative autoradiography using radiolabelled PGE2 (Matsumura et al., 1990, Matsumura et al., 1992). This fact enhances the reliability of our immunohistochemical investigation and suggests that the current results show the distribution of functional EP3R. Furthermore, the high similarity indicates that many of the CNS actions of PGE2 may be ascribable to EP3R among the four PGE receptor subtypes.
We observed neuronal cell bodies exhibiting EP3R-LI in several monoamine-containing nuclei. Among them, the locus coeruleus and subcoeruleus nucleus contain A6 and A7 noradrenaline cell groups, respectively. In the locus coeruleus, EP3R appears to be located on somatodendritic plasma membrane, because many fine processes and a few neuronal cell bodies with EP3R-LI were densely localized, and a previous in situ hybridization study showed intense signals for mouse EP3R mRNA in the nucleus (Sugimoto et al., 1994). Because the locus coeruleus, through its massively divergent efferent projections, has been implicated in vigilance, alertness, orientation, and attention (for reviews, see Aston-Jones et al., 1991; Foote et al., 1991), EP3R may play a role in these attentional processes by regulating the inputs to the nucleus postsynaptically. In addition to the noradrenergic cell groups, neuronal cells with EP3R-LI were seen in most of the raphe nuclei, which contain serotonergic cell groups. Furthermore, scattered neuronal cell bodies showing EP3R-LI also were detected in the pontine tegmental reticular nucleus, where the B9 serotonin cell group is found. The distribution patterns of the neuronal cells with EP3R-LI in the nuclei are quite similar to the immunostaining patterns seen with anti-serotonin antibodies (Steinbusch, 1981; Törk, 1985). An electrophysiologic study showed previously that direct agonist activation of EP3R led to excitation of putative serotonergic neurons in the rat dorsal raphe nucleus (Momiyama et al., 1996). Therefore, it is possible that EP3R, when it is placed on the postsynaptic membrane of serotonin-containing neurons, modulates the activities of the neurons and, thus, affects the physiologic actions of the serotonin system, such as analgesia mediated by serotonergic neurons descending from the raphe magnus nucleus to the spinal cord (Mohrland and Gebhart, 1980; Vasko et al., 1984). In light of our previous observation of signals for EP3R mRNA in the compact part of the substantia nigra (Sugimoto et al., 1994), in which many dopamine-containing neurons are localized, dopaminergic neurons also may express EP3R. However, we could not detect EP3R-LI in the substantia nigra or the ventral tegmental area. In the caudate-putamen, one of the major dopaminergic projection sites from those regions, weak EP3R-LI was found. Thus, a small amount of EP3R protein possibly may be located on axon terminals projected from the dopaminergic neurons residing in the substantia nigra.
EP3R-LI was observed in some of the nuclei controlling the autonomic nervous system. In the medulla oblongata, neuronal cells with EP3R-LI were observed in the dorsal motor nucleus of the vagus nerve, which contains preganglionic neurons of the parasympathetic nervous system. Moreover, some neuronal cells and fine dendritic processes showing intense EP3R-LI were found in the external formation of the ambiguus nucleus, which was shown previously to contain parasympathetic preganglionic neurons innervating the heart and supradiaphragmatic structures (Bieger and Hopkins, 1987). At the sacral cord level, the parasympathetic preganglionic neurons also appeared to exhibit EP3R-LI. We could not identify the expression of EP3R in the other parasympathetic nuclei, including the salivatory nuclei and the Edinger-Westphal nucleus. In the sympathetic nervous system, preganglionic neurons are located in the intermediolateral nucleus of lower cervical and all thoracic segments. EP3R-LI was observed in the neuropil and in several neuronal cell bodies of the intermediolateral nucleus, suggesting that a population of the sympathetic preganglionic neurons express EP3R. These results indicate that EP3R may regulate the output mediated by those autonomic preganglionic neurons from the CNS. However, most of pharmacologic studies on the functions of PGE2 in the autonomic nervous system have focused thus far on the EP3R that is located on peripheral terminals of the autonomic postganglionic nerve fibers (Mantelli et al., 1991; Molderings et al., 1992, Molderings et al., 1994; Reinheimer et al., 1998; Spicuzza et al., 1998). Therefore, the distribution of EP3R-LI in this study may help to reveal the unknown roles of PGE2 in the central autonomic system.
The nucleus of the solitary tract showed intense EP3R-LI in the neuropil. Because EP3R-LI also was seen in the nodose ganglion and axons of the vagus nerve, it seems reasonable to suppose that EP3R is distributed on the central endings of the vagus nerve and that the presynaptic EP3R in the nucleus of the solitary tract modulates inflow of visceral sensory information through the vagus nerve. Other researchers recently showed that mRNAs for EP3R and interleukin-1 receptor were expressed within the rat nodose ganglion and that an increase in the discharge activity of gastric vagal afferents after administration of interleukin-1β was attenuated by pretreatment with indomethacin, an inhibitor of PG synthesis, suggesting that PG is necessary for part of the cytokine-induced activation of vagal sensory functions (Ek et al., 1998). Because activation of the vagal sensory system is thought to be necessary for the CNS response during local infections in the abdominal or thoracic cavities (Elmquist et al., 1997), it is likely that PGE2 plays an essential role in the immune response of the CNS through EP3R in the vagal afferent system. Lately, one of the other PGE receptor subtypes, EP4, was shown to be expressed in neurons of the nucleus of the solitary tract by in situ hybridization (Zhang and Rivest, 1999). Although it has not yet been clarified whether EP4 receptor protein is located in the nucleus or in the projection sites, PGE2 may exert dual modulatory functions in the transmission of visceral sensory information through the two different receptor subtypes.
It has been reported that intrathecal administration of PGE2 into mice induced hyperalgesic effects to noxious stimuli (Taiwo and Levine, 1988; Uda et al., 1990), and a part of this PGE2 action has been supposed to involve EP3R (Minami et al., 1994). However, the neuronal mechanism for the EP3R-mediated hyperalgesia remains obscure. The dorsal horn of the spinal cord is an important site for pain transmission, and we observed EP3R-LI in the neuropil of laminae I and II of the spinal dorsal horn throughout the full length of the spinal cord. This result is consistent with a recent study that examined only the lumbar spinal segments (Beiche et al., 1998). Furthermore, EP3R-LI also was observed in the dorsal root ganglia of all spinal segments, where both small and large neurons exhibited immunoreactivity, and the small neurons were the majority. This neuronal population seems to be in agreement with the distribution of EP3R mRNA in the mouse dorsal root ganglion (Sugimoto et al., 1994). Sensory neurons in the dorsal root ganglion terminate in laminae I and II of the spinal cord. There is sufficient evidence to show that the EP3R-LI observed in the dorsal horn is derived from the EP3R-expressing sensory neurons in the dorsal root ganglion. We have found that the intensity of the EP3R-LI in the dorsal horn was reduced substantially by unilateral dorsal rhizotomy (Yun-Qing Li, K.N., M.N., T.K., personal communication). Consonant with our observation, it was reported that unilateral dorsal rhizotomy decreased the density of tritiated PGE2 binding sites in the dorsal horn (Matsumura et al., 1995). Thus, EP3R most likely is located on the central endings of the primary afferent fibers of the sensory neurons, and it may modulate pain transmission and may be involved in PGE2-induced hyperalgesia.
The distribution pattern of EP3R-LI also implied the receptor's modulatory functions in the visual system. Immunoreactivity was detected in the neuropil of the lateral geniculate nucleus, olivary pretectal nucleus, nucleus of the optic tract, superficial gray and optic nerve layers of the superior colliculus, and lateral posterior thalamic nucleus, all of which are known to be innervated by retinal axons (for review, see Sefton and Dreher, 1995). In another line of evidence, an immunohistochemical report on porcine ocular tissues showed expression of EP3α receptor in ganglion cells of the retina (Zhao and Shichi, 1995). However, we could not find any immunoreactivity in the suprachiasmatic nucleus or terminal nuclei of the accessory optic tract, which also are retinorecipient nuclei, suggesting that a population of retinal ganglion cells does not express EP3R. In addition to vision, olfaction also may be modulated by EP3R. Although the olfactory bulb showed no EP3R-LI, mouse EP3R mRNA has been shown to be expressed in mitral cells of the main and accessory olfactory bulbs (Sugimoto et al., 1994). Moreover, EP3R-LI was detected in the neuropil of layer I of the piriform cortex, the superficial part of which receives most of the afferent fibers from the mitral cells directly. These results indicate that, in the visual and olfactory systems, the EP3R located on the central terminals of the sensory fibers may play some regulatory roles in the synaptic transmission conveying sensory information to the central processing system.
The thalamus, especially the anterior portion, is the most conspicuous for intense EP3R-LI in the neuropil among the regions examined in the current study, and many neuronal cell bodies in several thalamic nuclei also showed immunoreactivity. However, the roles of EP3R in the thalamus have not yet been discerned. It has been believed that the thalamus is the gateway to the cerebral cortex: It filters and transforms the flow of information from subcortical sites to the cortex (for review, see Sherman and Koch, 1998). The thalamic nuclei that were immunostained strongly belong to the “limbic” or “nonspecific” thalamus. Limbic thalamic nuclei, which include the anterior nuclear group and the mediodorsal nucleus, innervate the limbic cortices and the amygdaloid region. Nonspecific thalamic nuclei contain the intralaminar and midline nuclei, project to widespread areas of the cortex, and terminate in layers other than layer IV, often in layers I and V. This fact is interesting, because the laminar termination pattern matches somewhat the staining pattern in the cortex. Furthermore, the weakly stained thalamic nuclei tend to be the specific relay nuclei that focus their projections on immunonegative layer IV.
The nonspecific thalamic nuclei have been considered to form part of the morphologic substratum of the “ascending activating system,” which was assumed to determine the level of consciousness in all its variations from complete alertness and attention to drowsiness and sleep. Many physiologic studies have shown sleep-wake regulation by PGE2 (for review, see Hayaishi and Matsumura, 1995). Although the site of action of PGE2 for the regulation of sleep-wakefulness is known to be in the posterior hypothalamus (Onoe et al., 1992), it also is likely that PGE2 modulates the ascending activating system through the EP3R in the nonspecific thalamic nuclei and, thereby, affects the sleep-wake states.
Previously, we suggested that the EP3R located in the median preoptic nucleus and the medial preoptic area was involved in fever induction by PGE2 (Nakamura et al., 1999). The neuronal cell group exhibiting EP3R-LI in these preoptic regions seemed to expand into the lateral septal nucleus. A recent histochemical study in the rat brain showed that an intracerebroventricular injection of lipopolysaccharide, which evoked fever, induced cyclooxygenase-2 in endothelial cells of the blood vessels distributed in the lateral septal nucleus as well as in and around those preoptic regions (Cao et al., 1999). During inflammation, therefore, the PGE2 produced by the induced synthase may activate the EP3R on the neurons, which are located not only in those preoptic regions but also in the lateral septal nucleus, and, thus trigger the febrile responses in the CNS. Furthermore, EP3R-LI was observed in the ependyma surrounding the lateral ventricle, the third and fourth ventricles, and the aqueduct. Although the physiologic role of PGE2 in the ependyma remains unknown, it has been reported that the PGE2 level in cerebrospinal fluid rose after an administration of pyrogens (Coceani et al., 1988). Thus, it is possible that the PGE2 in cerebrospinal fluid binds to the EP3R on the surface of ependymal cells to mediate some types of peripheral immune stimuli to the CNS.
Synaptic localization of EP3R
Many pharmacologic studies have indicated that EP3R functions as a presynaptic receptor (Mantelli et al., 1991; Molderings et al., 1992, Molderings, et al., 1994; Exner and Schlicker, 1995; Reinheimer et al., 1998; Spicuzza et al., 1998). From the above-mentioned results of the dorsal rhizotomy experiments, it is inferred that the EP3R protein in the spinal dorsal horn is supplied through axons from dorsal root ganglion cells and is located on the presynaptic membrane. In the current study, EP3R-LI was found in the neuropil of some regions in which the expression of EP3R mRNA has not been reported, such as the superior colliculus and the nucleus of the solitary tract. We believe that these regions also contain presynaptic EP3R protein that originates from the primary sensory neurons that project into the regions. In the compact part of the dorsomedial hypothalamic nucleus, intense EP3R-LI was observed in the neuropil, but immunostained cell bodies were not detected. EP3R mRNA-expressing neurons were distributed broadly in the nucleus (Sugimoto et al., 1994). It is possible that the EP3R-expressing neurons in the nucleus project the axons into the compact part and that the receptor protein from the neurons is accumulated on the axon terminals. On the other hand, neuronal cell bodies and dendrites were immunostained in some other regions, such as the thalamus, medial preoptic area, raphe nuclei, and locus coeruleus. Therefore, EP3R appears to be located on postsynaptic membrane as well as presynaptic membrane. Immunoelectron microscopic analysis would certify the synaptic localization of the receptor protein and provide us with important information on the mechanisms for the synaptic functions of EP3R.
EP3R isoforms and functional significance
The antibody used in the current study was raised against a common portion to three alternatively spliced isoforms—EP3α, EP3β, and EP3γ—which are different only in their C-terminal tails (Takeuchi et al., 1993, Takeuchi, et al., 1994; Kitanaka et al., 1996). Immunohistochemical analyses using antibodies against the distinctive portions of the EP3R isoforms would reveal isoform-specific distribution patterns. Recently, it was shown with metabotropic glutamate receptors that axon/dendrite targeting of the receptors was determined by their C-terminal domains (Stowell and Craig, 1999). We believe that, as discussed above, EP3R works as both a presynaptic receptor and a postsynaptic receptor and that it may have different synaptic regulatory mechanisms suitable for its location. These distinct synaptic localizations and functions of EP3R may be ascribed to the spliced isoforms. In other words, each isoform may show its specific synaptic localization and function.
We have cloned complementary DNAs of four bovine EP3R isoforms—EP3A, EP3B, EP3C, and EP3D—and have shown that they are coupled to different G-proteins in a nonneuronal cell line (Namba et al., 1993). This indicates the possibility that the EP3R isoforms regulate synaptic transmission through different intracellular signaling pathways in the nervous system. We recently reported that the EP3B receptor was involved in the regulation of neurotransmitter release by a novel mechanism. Activation of the receptor inhibited dopamine release from a neuroendocrine cell line without affecting Ca2+ mobilization in the cells, suggesting that the EP3B receptor-mediated inhibition of neurotransmitter release may be caused at a step after the Ca2+ mobilization, such as fusion of secretory granules and plasma membrane (Nakamura et al., 1998). Further studies may reveal the other various mechanisms for the synaptic modulation mediated by the EP3R isoforms.
CONCLUSIONS
This is the first study on overall distribution of PGE receptor proteins in the nervous system. EP3R-LI was observed in various regions, and its distribution suggested unknown CNS functions of EP3R, such as modulation of the noradrenergic and serotonergic monoamine systems. The results of this study also imply the involvement of EP3R in many physiologic actions of various nervous systems, such as autonomic control of homeostasis, somatic and visceral sensations, olfaction, vision, and fever induction. Taken together, these results imply that EP3R may play modulatory roles in “central-peripheral transmission.” Furthermore, other unidentified functions of EP3R may be discovered by future studies in light of the results shown in this study. The documented distribution of EP3R will contribute to a further understanding of the diverse actions of PGE2 in the central and peripheral nervous systems.
Acknowledgements
The authors are grateful to Drs. Satoshi Tanaka, Yun-Qing Li, and Jin-Lian Li for their helpful technical advice and to Mr. Akira Uesugi for his photographic support. This work was supported in part by grants in aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan. K.N. is the recipient of a research fellowship from the Japan Society for the Promotion of Science for Young Scientists.




