Distribution of CGRP-like immunoreactivity in the chick and quail brain
Abstract
Calcitonin gene-related peptide (CGRP)-containing neurones have been implicated in the transmission of visceral sensory information to the cortex and in the control of arterial blood pressure in mammals. However, little is known about its function in other vertebrates. As a first step toward investigating the function of CGRP in birds, its distribution was studied in the domestic chick and quail brain by means of immunocytochemistry, by using antibodies against rat CGRP. The distribution of CGRP immunoreactivity in the chick and quail central nervous system was found to be similar. CGRP-immunoreactive (CGRPi) perikarya were not present in the telencephalon. In the diencephalon, CGRPi perikarya were present mainly in the shell of the thalamic nucleus ovoidalis, the nucleus semilunaris paraovoidalis, the nucleus dorsolateralis posterior thalami, and in the hypothalamic nucleus of the ansa lenticularis. In the brainstem, CGRPi perikarya were present in the nucleus mesencephalicus nervi trigemini, the nucleus tegmenti ventralis, the locus coeruleus, the nucleus linearis caudalis and in the parabrachial region. In addition CGRPi perikarya were found in the motor nuclei of the III, IV, V, VI, VII, IX, X, and XII cranial nerves. The telencephalon contained CGRPi fibres within the paleostriatal complex (mainly in the ventral paleostriatum), parts of the neostriatum and ventral hyperstriatum, parts of the archistriatum, and the septum. In the diencephalon, the densest plexus of CGRPi fibres was observed in the dorsal reticular thalamus. A less dense CGRPi innervation was present in some dorsal thalamic nuclei and in the medial and periventricular hypothalamus. The pretectum and midbrain tegmentum also contained CGRPi fibres, whereas the optic tectum was virtually devoid of immunolabelling. Scattered CGRPi fibres were observed in the central grey and neighbouring pontine areas. Some of the sensory fibres of the trigeminal, vagal, glossopharyngeal, and spinal nerves were also CGRPi. The results of comparative studies indicate that the presence of CGRP in some thalamo-telencephalic projections is a primitive feature of the forebrain of amniotes. Therefore, the brain areas giving rise to and receiving such a projection in different vertebrates, are likely to be homologous. J. Comp. Neurol. 421:515–532, 2000. © 2000 Wiley-Liss, Inc.
Calcitonin gene-related peptide (CGRP) is a 37 amino acid peptide that is formed by alternative RNA processing of the calcitonin gene (Amara et al., 1982). In the nervous system, CGRP was first demonstrated to be present in rat primary sensory neurones and in neurones of some cranial nerve motor nuclei and brainstem nuclei (Rosenfeld et al., 1983). Subsequent investigations (Gibson et al., 1984; Kawai et al., 1985; Skofitsch and Jacobowitz, 1985; Harmann et al., 1988; Kruger et al., 1988a,Kruger et al., 1988b; Sugimoto et al., 1988), have revealed that the distribution of CGRP in the central nervous system of mammals is much more extensive than originally reported.
The role of CGRP-containing neurones in the nervous system is not well established. In mammals, they have been suggested to be involved in the transmission of visceral sensory information to the cerebral cortex (Shimada et al., 1985a,Shimada et al., 1985b; Yasui et al., 1989). Injection of CGRP into the central nucleus of the amygdala has also been shown to elicit increases in arterial blood pressure and heart rate (Nguyen et al., 1986). In contrast, intravenous administration of CGRP has been demonstrated to decrease mean arterial pressure (Fisher et al., 1983). Thus, CGRP-containing neurones may play a variety of roles, a view supported by their extensive distribution throughout the rat central nervous system (Kawai et al., 1985).
There is little information about the distribution of CGRP in the brain of nonmammals. Brauth and Reiner (1991) studied the distribution of CGRP in the forebrain of rats, pigeons, crocodiles, and turtles and concluded that it is a well-conserved marker of the amniote auditory thalamo-telencephalic pathway. They also described CGRP-immunoreactive (CGRPi) fibres and terminal fields to be present in basal telencephalic structures of pigeons, crocodiles, and turtles (Brauth and Reiner, 1991). Although a more recent report described the sexually dimorphic distribution of CGRP-immunoreactive fibres and perikarya in several song-related telencephalic nuclei in the zebra finch (Bottjer et al., 1997), to our knowledge, a detailed description of the distribution of CGRP immunoreactivity in the avian brain has hitherto been unavailable. Therefore, the distribution of CGRP immunoreactivity was investigated throughout the brains of two frequently studied avian species, the domestic chicken and the Japanese quail. The information obtained may help to shed light on the organisation of the avian brain and the significance and comparative evolution of CGRP-containing neural systems.
Abbreviations
-
- III
-
oculomotor nerve
-
- V
-
trigeminal nerve
-
- VIIIc
-
nervus octavus, pars cochlearis
-
- VIIIv
-
nervus octavus, pars vestibularis
-
- IX
-
glossopharyngeal nerve
-
- X
-
vagus nerve
-
- AA
-
anterior archistriatum
-
- Ac
-
nucleus accumbens
-
- AL
-
ansa lenticularis
-
- ALA
-
nucleus ansae lenticularis anterior
-
- AId
-
archistriatum intermedium pars dorsalis
-
- AIv
-
archistriatum intermedium pars ventralis
-
- ALP
-
nucleus ansae lenticularis posterior
-
- Am
-
archistriatum mediale
-
- AM
-
nucleus anterior medialis hypothalami
-
- Amb
-
nucleus ambiguus
-
- An
-
nucleus angularis
-
- AP
-
area pretectalis
-
- Ap
-
archistriatum posterior
-
- AVT
-
ventral tegmental area (Tsai)
-
- BCS
-
brachium colliculi superioris
-
- BNST
-
bed nucleus of the stria terminalis
-
- BNSTl
-
BNST, pars lateralis
-
- BNSTm
-
BNST, pars medialis
-
- CA
-
commissura anterior
-
- CbL
-
nucleus cerebellaris internus
-
- CGRP
-
calcitonin gene-related peptide
-
- CGRPi
-
calcitonin gene-related peptide immunoreactive
-
- CE
-
nucleus cuneatus externus
-
- CPi
-
cortex piriformis
-
- D
-
nucleus of Darkschewitsch
-
- DIP
-
nucleus dorsointermedius posterior thalami
-
- DIVA
-
nucleus dorsalis intermedius ventralis anterior
-
- DLL
-
nucleus dorsolateralis anterior thalami, pars lateralis
-
- DLM
-
nucleus dorsolateralis anterior thalami, pars medialis
-
- DLP
-
nucleus dorsolateralis posterior thalami
-
- DMA
-
nucleus dorsomedialis anterior thalami
-
- DMP
-
nucleus dorsomedialis posterior thalami
-
- DSM
-
decussatio supramamillaris
-
- E
-
ectostriatum
-
- EW
-
nucleus of Edinger-Westphal
-
- FA
-
tractus fronto-archistriaticus
-
- FL
-
field L of the neostriatum
-
- FLM
-
fasciculus longitudinalis medialis
-
- FPL
-
fasciculus prosencephali lateralis
-
- FRL
-
formatio reticularis lateralis mesencephali
-
- FRM
-
formatio reticularis medialis mesencephali
-
- GC
-
nucleus gracillis
-
- GCt
-
substantia grisea centralis
-
- GLDp
-
nucleus geniculatus lateralis, pars dorsalis principalis
-
- GLv
-
nucleus geniculatus lateralis, pars ventralis
-
- HA
-
hyperstriatum accessorium
-
- HD
-
hyperstriatum dorsale
-
- HIS
-
hyperstriatum intercalatum supremum
-
- HL
-
nucleus habenularis lateralis
-
- HM
-
nucleus habenularis medialis
-
- Hp
-
hippocampus
-
- HV
-
hyperstriatum ventrale
-
- ic
-
nucleus intercalatus
-
- ICo
-
nucleus intercollicularis
-
- ICT
-
nucleus intercalatus thalami
-
- IH
-
nucleus inferioris hypothalami
-
- Imc
-
nucleus isthmi pars magnocellularis
-
- INP
-
nucleus intrapeduncularis
-
- IO
-
nucleus isthmo-opticus
-
- IP
-
nucleus interpeduncularis
-
- Ipc
-
nucleus isthmi pars parvocellularis
-
- La
-
nucleus laminaris
-
- LC
-
nucleus linearis caudalis
-
- LFS
-
lamina frontalis superior
-
- LFSM
-
lamina frontalis suprema
-
- LH
-
lamina hyperstriatica
-
- LHy
-
regio lateralis hypothalami
-
- LL
-
nucleus lemnisci lateralis
-
- LMD
-
lamina medullaris dorsalis
-
- LMmc
-
nucleus lentiformis mesencephali, pars magnocellularis
-
- LMpc
-
nucleus lentiformis mesencephali, pars parvocellularis
-
- LoC
-
locus coeruleus
-
- LPO
-
lobus parolfactorius
-
- MCC
-
nucleus magnocellularis cochlearis
-
- ML
-
nucleus mamillaris lateralis
-
- MLd
-
nucleus mesencephalicus lateralis, pars dorsalis
-
- MnV
-
nucleus motorius nervi trigemini
-
- MnVII
-
nucleus motorius nervi facialis
-
- MnVIId
-
nucleus motorius nervi facialis, pars dorsalis
-
- MnVIIv
-
nucleus motorius nervi facialis, pars ventralis
-
- N
-
neostriatum
-
- nBOR
-
nucleus opticus basalis
-
- NC
-
neostriatum caudale
-
- Ndc
-
neostriatum dorsocentrale (Metzger et al., 1998)
-
- Ndl
-
neostriatum dorsolaterale (Metzger et al., 1998)
-
- NI
-
neostriatum intermedium
-
- nIII
-
nucleus nervi oculomotorii
-
- nIV
-
nucleus nervi trochlearis
-
- nIX
-
nucleus nervi glossopharyngei
-
- nIX-X
-
nucleus nervi glossopharingei et nucleus motorius dorsalis nervi vagi
-
- nVM
-
nucleus mesencephalicus nervi trigemini
-
- nVI
-
nucleus nervi abducentis
-
- nX
-
nucleus dorsalis nervi vagi
-
- nXII
-
nucleus nervi hypoglossi
-
- OI
-
nucleus olivaris inferior
-
- OS
-
nucleus olivaris superior
-
- Ov
-
nucleus ovoidalis
-
- Ov (s)
-
shell of nucleus ovoidalis
-
- OM
-
tractus occipitomesencephalicus
-
- PA
-
paleostriatum augmentatum
-
- PB
-
nucleus parabrachialis
-
- PM
-
nucleus pontis medialis
-
- PMI
-
nucleus paramedianus internus thalami
-
- PMM
-
nucleus premamillaris
-
- PP
-
paleostriatum primitivum
-
- PPC
-
nucleus principalis precommissuralis
-
- PrV
-
nucleus sensorius principalis nervi trigemini
-
- PT
-
nucleus pretectalis
-
- PTM
-
nucleus pretectalis medialis
-
- PVH
-
nucleus periventricularis hypothalami
-
- PVN
-
nucleus paraventricularis magnocellularis (paraventricular nucleus)
-
- PVO
-
organum periventriculare
-
- PVT
-
paleostriatum ventrale
-
- QF
-
tractus quintofrontalis
-
- R
-
nucleus raphes
-
- Rgc
-
nucleus reticularis gigantocellularis
-
- ROT
-
nucleus rotundus
-
- RPaM
-
nucleus reticularis paramedianus
-
- RPgc
-
nucleus reticularis pontis caudalis pars gigantocellularis
-
- RPgl
-
nucleus reticularis paragigantocellularis lateralis
-
- RSd
-
nucleus reticularis superior, pars dorsalis
-
- RST
-
nucleus reticularis subtrigeminalis
-
- RSv
-
nucleus reticularis superior, pars ventralis
-
- Ru
-
nucleus ruber
-
- S
-
nucleus tractus solitarii
-
- SCbd
-
tractus spinocerebellaris dorsalis
-
- SCd
-
nucleus subceruleus dorsalis
-
- SCv
-
nucleus subceruleus ventralis
-
- SCE
-
stratum cellulare externum
-
- SG
-
substantia gelatinosa Rolandi (trigemini)
-
- SL
-
lateral septum
-
- SLu
-
nucleus semilunaris
-
- SM
-
medial septum
-
- SN
-
substantia nigra
-
- SP
-
nucleus subpretectalis
-
- SPC
-
nucleus superficialis parvocellularis
-
- SpL
-
nucleus spiriformis lateralis
-
- SpM
-
nucleus spiriformis medialis
-
- SPO
-
nucleus paraovoidalis semilunaris
-
- SPOc
-
nucleus paraovoidalis semilunaris caudalis
-
- SRt
-
nucleus subrotundus
-
- SS
-
nucleus supraspinalis
-
- T
-
nucleus triangularis
-
- Ta
-
nucleus tangentialis
-
- TDV
-
nucleus et tractus descendens nervi trigemini
-
- Tn
-
nucleus taeniae
-
- TIO
-
tractus isthmo-opticus
-
- TOV
-
tractus ovoidalis
-
- ToS
-
torus semicircularis
-
- Pc
-
nucleus tegmenti pedunculopontinus, pars compacta
-
- TPO
-
area temporo-parieto-occipitalis
-
- TSM
-
tractus septomesencephalicus
-
- TT
-
tractus tectothalamicus
-
- TTS
-
tractus thalamostriaticus
-
- TV
-
nucleus tegmenti ventralis
-
- TVM
-
tractus vestibulomesencephalicus
-
- v
-
lateral ventricle
-
- VeD
-
nucleus vestibularis descendens
-
- VeL
-
nucleus vestibularis lateralis
-
- VeM
-
nucleus vestibularis medialis
-
- VeS
-
nucleus vestibularis superior
-
- VMN
-
nucleus ventromedialis hypothalami
-
- VP
-
ventral pallidum
MATERIALS AND METHODS
Eight domestic chicks (Gallus domesticus), between 3 and 10 days old, were used in this study. Four adult quail (Coturnix japonica), two males and two females, were also used to investigate possible interspecies differences in the distribution of CGRP within the avian brain. Throughout the experimental work, the birds were treated according to the European Communities Council Directives of November 24, 1986 (86/609/EEC).
Each bird was given a lethal intraperitoneal injection (0.4 ml/100 g body weight) of anaesthetic (Equithesin; Davies and Horn, 1983) and was perfused transcardially with normal saline followed by 150 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) at room temperature. In an attempt to enhance the concentration of intracellular CGRP, two chicks received an injection of 2.5 μl of an aqueous solution containing 5 μg/μl of colchicine (Sigma, St. Louis, MO) 24–48 hours before perfusion. The chicks were anaesthetised (Equithesin; 0.3 ml/100 g body weight, i.p.) and the injection placed in the lateral ventricle, by using a Hamilton microsyringe. After perfusion, the brains of all birds were removed and immersed in fixative similar to that used for perfusion, for 4–6 hours at 4°C. Subsequently, each brain was immersed in a solution of 30% sucrose in phosphate buffer at 4°C until it sank. Serial, coronal, or sagittal 30- or 40-μm-thick sections were then cut from each brain with a freezing microtome and collected into six matching series which were either processed immediately or frozen for subsequent use. Alternate parallel series were processed for CGRP immunohistochemistry and for Nissl staining (1% acidic toluidine blue), respectively. This facilitated the accurate cytoarchitectonic localisation of the CGRP-immunoreactive (CGRPi) structures.
The tissue sections were processed for immunocytochemistry by using the avidin-biotin complex method. To enhance penetration, the antibodies and the avidin-biotin complex were diluted in Tris buffered saline (pH 7.6) containing 0.3% Triton-X-100 (Sigma). Before immunolabelling, endogenous peroxidase activity in the tissue sections was suppressed by incubation in a 1% H2O2 solution for 30 minutes. The sections were then incubated overnight at 4°C in anti-rat CGRP raised in rabbit (Sigma) diluted 1:4,000 in Tris buffered saline containing 0.3% Triton-X-100 and 5% normal goat serum. The sections were then incubated in biotinylated goat-anti rabbit immunoglobulin G (Vector Laboratories, Burlingame, CA) diluted 1:200 in Tris buffered saline containing 0.3% Triton-X-100 for 2 hours at room temperature, followed by a further 2 hours in the avidin-biotin Elite complex (Vectastain Elite kit, Vector Labs) diluted in similar buffer. The resulting peroxidase labelling was visualised by using 0.04% diaminobenzidine (Sigma) in Tris buffer, pH 7.6 with 0.01% H2O2 as a chromogen. The sections were then mounted onto subbed glass slides, air-dried, dehydrated through a series of graded alcohols, cleared in xylene, mounted in Permount (Fisher, Fair Lawn, NJ), and cover-slipped. Control sections were treated identically to the immunolabelled sections except for omission of the primary antibody or its substitution by rabbit nonimmune serum.
RESULTS
The CGRP antibody used in the present study gave rise to qualitatively similar immunolabelling in the chick and quail brain and the distribution of CGRPi perikarya and fibres was similar in the two species. Although colchicine has previously been reported to enhance the CGRP immunoreactivity of neuronal perikarya (Brauth and Reiner, 1991), no difference was observed between the immunolabelling in colchicine-treated and untreated chicks in the current study. Omission of the primary antibody or its substitution by rabbit nonimmune serum abolished the CGRP immunolabelling.
Despite that background labelling was generally low, some brain regions displayed faint nongranular labelling of perikarya, which contrasted with the faint-granular or dense-diffuse immunolabelling of perikarya in other brain areas. Faint nongranular staining of perikarya was observed in both the magnocellular and parvocellular parts of the isthmic nucleus, the nucleus isthmo-opticus, the nucleus semilunaris and some large neurones in the nucleus laminaris and nucleus magnocellularis cochlearis. Some of the Purkinje cells of the cerebellar cortex also displayed nongranular labelling, as has been reported for mammals (Kawai et al., 1985; Kruger et al., 1988b). The significance of this nongranular perikaryonal labelling is unclear, but in view of its faint and diffuse appearance and its variability within the same brain (for instance, the labelling intensity of Purkinje cells varied), such labelled structures have not been included in our charting of immunoreactive cell bodies (Fig. 1).
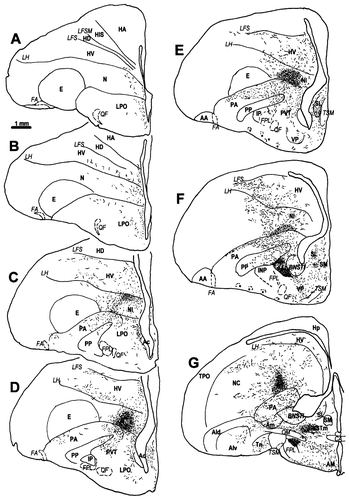
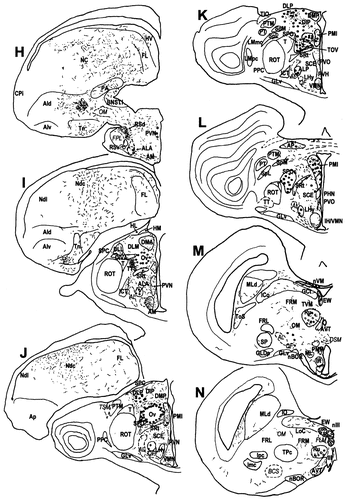
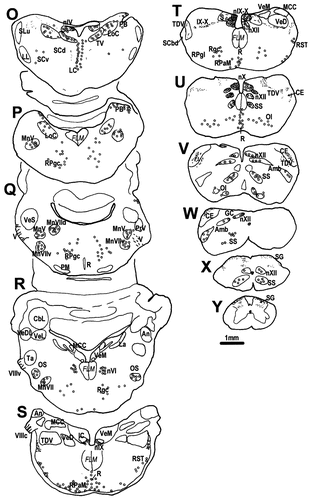
A–Y: A diagrammatic representation of the distribution of CGRPi perikarya and fibres in the brain of the chick. Filled circles represent densely labelled perikarya, whereas open circles represent perikarya showing faint, usually granular labelling. Lines and dots indicate the distribution of CGRPi fibres and terminals. Stars in E and F indicate the basket-like innervation of the ventral PA, which are much more distinctive in quail than in chicks. For abbreviations, see list. Scale bar = 1 mm.
The neuroanatomic nomenclature of Kuenzel and Masson (1988) for the chick brain has been adopted and modified where necessary in the light of subsequent investigations of the anatomy and neurochemistry of the avian brain.
Distribution of CGRP-immunoreactive perikarya
The immunohistochemical protocol described above resulted in immunolabelling of perikarya in the same areas of the central nervous system of chicks and quail. However, in general, the density of the immunolabelling within the perikarya was lower in quail than in chick.
Forebrain.
CGRPi perikarya were not observed in the telencephalon. Immunopositive perikarya were present in the thalamus, forming a shell around the nucleus ovoidalis (Ov; and possibly in the periphery of the nucleus itself) and extending ventrolaterally into an area that we have tentatively named the nucleus semilunaris paraovoidalis (SPO; Figs. 1J,K, 2A–D), in light of the work of Karten and Hodos (1967) and Wild et al. (1993) on the pigeon and Metzger et al. (1998) on the chick. Caudally, immunolabelled perikarya in the SPO extended into an ill-defined area just medial to the caudal aspect of the nucleus rotundus, that we refer to as the caudal SPO (SPOc; Figs. 1L, 2B). Another immunoreactive cell group was present within the dorsolateral posterior thalamic nucleus (DLP), where many cells showed strong immunoreactivity (Figs. 1J,K, 2A,B,E). The nucleus dorsointermedius posterior thalami (DIP) also contained a few scattered CGRPi perikarya. More caudally, several CGRPi perikarya were present in the nucleus paramedianus internus thalami (PMI) (Figs. 1L, 2D).
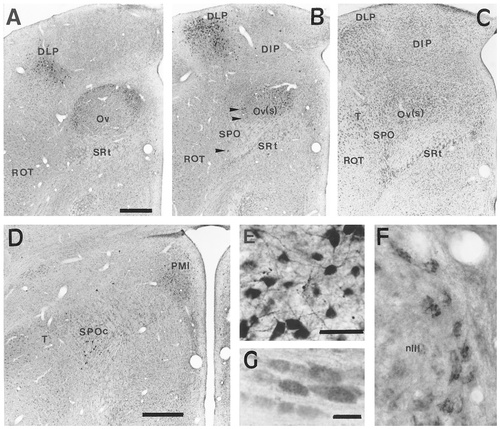
The distribution of CGRPi perikarya in the brain of the chick and quail. A: Low-power photomicrograph of a coronal section through the dorsal thalamus of a chick at intermediate-to-rostral levels, showing CGRPi perikarya in the shell of nucleus ovoidalis (Ov) and in the rostral aspect of the dorsolateral posterior thalamic nucleus. A few CGRPi perikarya are also visible in the nucleus semilunaris paraovoidalis (SPO). B: Low-power photomicrograph of a coronal section through the dorsal thalamus of a chick at intermediate-to-caudal levels, showing CGRPi perikarya in the ovoidalis shell [Ov(s)] capping the nucleus ovoidalis (Ov) caudally, and in the DLP. Scattered CGRPi perikarya are also visible in the nucleus dorsointermedius posterior thalami (DIP). Arrowheads point to CGRPi perikarya in an area just lateral and caudal to Ov. C: Photomicrograph of a Nissl-stained coronal section of the dorsal thalamus of a chick, adjacent to that shown in B, illustrating the cytoarchitecture of the nuclei that display CGRPi perikarya. Note the presence of a conspicuous cell group just lateral to the Ov and medial to nucleus triangularis, which shows a few CGRPi perikarya (arrowheads in B). D: Photomicrograph of a coronal section of the chick brain at the thalamo-pretectal boundary, showing CGRPi perikarya in the nucleus paramedianus internus (PMI) and just medial to the caudal edge of the nucleus rotundus and nucleus triangularis. Here, immunolabelled perikarya apparently constitute the caudal continuation (SPOc) of the CGRPi perikarya in the SPO. E: Photomicrograph illustrating the nongranular labelling in the perikarya and dendrites of cells in the DLP, that is typical of the labelling of CGRPi forebrain neurons. F: Photomicrograph showing CGRPi perikarya in the nucleus of the third cranial nerve (oculomotorius), illustrating the granular labelling typical of many motoneurons in the midbrain and brainstem. G: High-power photomicrograph of the CGRPi perikarya in the nucleus mesencephalicus nervi trigemini, showing diffuse, granular labelling. For abbreviations, see list. Scale bars = 50 μm in A (applies to A–C), in D, and in E (applies to E,F); 37.5 μm in G.
In the hypothalamus, CGRPi cells were only present in the nucleus of the ansa lenticularis (Fig. 1H–M), where they constituted a sparse population of relatively faintly immunolabelled neurones. A few faintly immunoreactive neurones were also observed in the rostral nucleus paraventricularis magnocellularis (Fig. 1H).
Midbrain and brainstem.
CGRPi neurones in the midbrain and brainstem generally displayed a fainter labelling than those in the forebrain. Moreover, many of them showed a granular reaction product distributed over but confined to the perikaryon, thus, differing from diencephalic CGRPi cells, in which dense labelling was distributed diffusely over the perikaryon and extended into the principal dendrites (Fig. 2E). This granular pattern of labelling was typical, but not specific, to neurones in the motor nuclei of the cranial nerves (Fig. 2F).
The most rostral CGRPi neurones in the midbrain were found in the nucleus mesencephalicus nervi trigemini (Fig. 1M). These neurones showed dense, granular cytoplasmic labelling (Fig. 2G) and were principally located within the tectal commissure, but some were also present in the inner layers of the rostromedial optic tectum (not shown). More caudally, labelling was observed in some of the motoneurons of the oculomotor nerve nucleus (Figs. 1N, 2F), including the nucleus of Edinger-Westphal. At a similar level, granular labelling was observed over the cytoplasm of the large neurones in the red nucleus (Fig. 1N).
At pontine levels, CGRPi neurones showing granular labelling were present in the nucleus of the trochlear nerve, the nucleus tegmenti ventralis, and the locus coeruleus (LoC; Fig. 1O). Moreover, a few neurones showing granular labelling were observed dorsal and lateral to the LoC (Fig. 1P) in an area that seems to correspond to the superficial lateral and ventromedial nuclei of the parabrachial complex (Wild et al., 1990). Neurones showing granular labelling were also visible in the nucleus linearis caudalis, as well as scattered throughout the subcoerulear area (Fig. 1O).
Scattered immunolabelled perikarya were present in the pontine and medullary reticular formation, including the nuclei gigantocellularis, paragigantocellularis, and subtrigeminalis (Fig. 1P–T). Faintly reactive neurones showing granular labelling were also found just deep to the spinal lemniscus, in the medial and lateral pons (Fig. 1Q–T). Moreover, the superior (Fig. 1R) and inferior olivary nuclei (Fig. 1V) also displayed abundant CGRPi perikarya.
Most of the immunoreactive perikarya in the brainstem were located in motor nuclei. The trigeminal (MnV) and facial nerve (both dorsal and ventral parts; MnVII) (Fig. 1P,Q) as well as the nucleus of the nervus abducens (nVI) (Fig. 1R) were characteristically strongly immunopositive. More caudally, neurones belonging to the motor nuclei of the vagal and glossopharyngeal nerves also displayed labelled perikarya (Fig. 1S,T). These included not only the nucleus motorius nervi glossopharyngei (nIX) and the nucleus motorius dorsalis nervi vagi (nX), but also the nucleus centralis medullae oblongatae (Fig. 1V), which Wild et al. (1990) considered to represent the avian nucleus ambiguus (Amb). At medullary levels, immunolabelled perikarya were present in the nuclei that constitute the motor complex of the hypoglossal nerve, namely the nucleus nervi hypoglossi (nXII) and the nucleus supraspinalis (SS, see Dubbeldam, 1998; Fig. 1U–X).
Distribution of CGRPi fibres
Telencephalon.
Most of the CGRPi fibres entering the telencephalon coursed through the dorsal aspect of the fasciculus prosencephali lateralis (FPL, lateral forebrain bundle). Scattered, labelled fibres were also observed to run dorsal to the FPL and enter the telencephalon caudal and dorsal to the anterior commissure. Moreover, scattered fibres in the preoptic hypothalamus were observed to enter the basal telencephalon medially. However, CGRPi fibres were not observed in the tractus septomesencephalicus, the tractus occipitomesencephalicus, the tractus quintofrontalis, or in the pallial and anterior commissures.
Immunolabelled fibres could be followed within the telencephalic hemispheres and a series of CGRPi terminal fields was associated with them. However, several telencephalic areas seemed free of immunolabelling, including the olfactory bulbs, hyperstriatum accessorium, hyperstriatum intercalatum supremum, hyperstriatum dorsale, nucleus basalis, ectostriatum, hippocampus, area parahippocampalis, area corticoidea dorsolateralis, and the cortex piriformis.
CGRPi fibres entering the telencephalon from the medial preoptic hypothalamus were associated with a series of fibre fields in the medial aspect of the basal telencephalon (Fig. 1A–G), including the nucleus of the diagonal band and the septum. Immunolabelled fibres in the septum were mainly located in its lateral part (SL; Fig. 1D–F), although caudally they extended into the medial septum (Figs. 1F, 3A,E). Most of the CGRPi innervation of the septum seemed to terminate as perisomatic baskets surrounding unlabelled perikarya. At rostral levels, the fibres that enter the basal telencephalon medially seem to contribute to the innervation of the medial lobus parolfactorius (LPO) next to the nucleus accumbens (Fig. 3B). At precommissural levels, CGRPi fibres were observed in the basal telencephalon ventral to the FPL (Fig. 1E,F), where they formed complex basket-like figures surrounding unlabelled somata. These CGRPi “baskets” were more prominent in quail than in chicks.
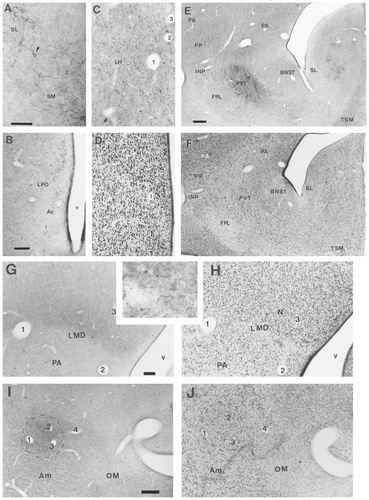
The distribution of CGRPi fibres in the telencephalon of the chick. A: Photomicrograph of CGRPi fibres in the lateral and medial septum of a chick, in close apposition to the perikarya and proximal dendrites of septal neurons. B: Photomicrograph of CGRPi fibres in the rostromedial lobus paraolfactorius (LPO), including the presumptive nucleus accumbens (Ac). C: Photomicrograph of CGRPi fibres adjacent to the lamina hyperstriatica (LH) at intermediate-to-rostral levels of the telencephalon. 1,2, and 3 mark blood vessels. D: Photomicrograph of a Nissl-stained section adjacent to that shown in C, illustrating the cytoarchitecture of the border between the anterior neostriatum (N) and ventral hyperstriatum (HV). The numbers mark the blood vessels identified in C. E: Low-power photomicrograph of a coronal section through the intermediate-to-caudal telencephalon of a chick, showing the dense CGRPi terminal field in the paleostriatum ventrale (PVT) and the CGRPi baskets in the septum. F: Low-power photomicrograph of a Nissl-stained coronal section adjacent to that shown in E, illustrating the cytoarchitecture of the PVT and neighbouring areas in the basal ganglia. G: Photomicrograph of a coronal section of the chick brain showing the CGRPi terminal field lying above the lamina medullaris dorsalis (LMD) in the intermediate to caudal part of the medial neostriatum. Inset: A higher power photomicrograph of the CGRPi fibres and terminals in the intermediate neostriatum. H: Photomicrograph of a Nissl-stained coronal section adjacent to that shown in G. The numbers in G and H indicate the same blood vessels. I: Photomicrograph of a coronal section of the chick brain showing a CGRPi terminal field in the medial archistriatum. J: Photomicrograph of a Nissl-stained coronal section adjacent to that shown in I. The blood vessels numbered in I and J, help to identify a recognisable nucleus in Nissl-stained material, which corresponds to the terminal CGRPi field shown in I. For abbreviations, see list. Scale bars = 100 μm in A (applies to A,C), in B (applies to B,D), in E (applies to E,F), and in G (applies to G,H,inset); 200 μm in I (applies to I,J).
Some of the CGRPi fibres travelling in the dorsal FPL seemed to innervate the lateral LPO (Fig. 1B,C) and give rise to relatively dense terminal fields in the paleostriatal complex and the intermediate and caudal parts of the hyperstriatum ventrale and neostriatum. At intermediate rostrocaudal levels of the telencephalon, CGRPi fibres were abundant in the medial paleostriatal complex (Fig. 1E,F). A denser CGRPi innervation was present in the dorsal aspect of the paleostriatum augmentatum (PA) just ventral to the lamina medullaris dorsalis (Fig. 1E,F). The paleostriatum ventrale (PVT, as defined by Kitt and Brauth, 1981) displayed the densest CGRPi terminal field in the telencephalon (Fig. 3E,F). Many fine labelled fibres crossed the lamina medullaris dorsalis (LMD) to develop a dense terminal field in the medial neostriatum (including the neostriatum intermedium, NI) (Figs. 1C–G, 3G,H), composed of very thin fibres and terminals (see inset in Fig. 3G,H). Thick CGRPi fibres crossed the LMD to reach the neostriatum and hyperstriatum ventrale, where they extended both rostrally and caudally. In the rostral telencephalon (Fig. 1A,B) sparse labelled fibres seemed to run in association with the lamina hyperstriatica (LH) (Fig. 3C,D) and lamina frontalis superior (LFS, Fig. 1C–E) to terminate sparsely in both the N and HV. These thick CGRPi fibres developed a relatively dense innervation of the caudal aspect of the N and HV (Fig. 1F–I). In the caudal neostriatum CGRPi fibres were mainly located in the neostriatum dorsocaudale, as defined in the domestic chick by Metzger et al. (1998), but both the field L of the N and the neostriatum dorsolaterale (again following Metzger et al., 1998) remained relatively free of labelling (Fig. 1I,J).
The archistriatum displayed a heterogeneous distribution of CGRPi fibres. The densest CGRPi terminal field was observed in the archistriatum mediale (Am; Figs. 1H, 3I,J). Scattered, labelled fibres were also present in the dorsal (AId) and ventral (Aiv) parts of the archistriatum intermedium, in the nucleus taeniae (Tn) where fibres were especially thick, and to a lesser extent in the archistriatum posterior (Ap). Some CGRPi fibres were seen to enter the archistriatal complex from the FPL. However, scattered immunolabelled fibres appeared to gain access to the archistriatum from the CGRPi fibre fields in the caudal paleostriatal complex, as well as from the bed nucleus of the stria terminalis (BNST; Fig. 1G), including it medial and lateral parts (Asté et al., 1998). Many of the CGRPi fibres extending into the caudal paleostriatal complex and BNST appeared to enter the telencephalon directly from the diencephalon, just dorsal to the anterior commissure.
Hypothalamus.
CGRPi fibres were present throughout the hypothalamus, although their density was invariably higher in periventricular and medial compared with lateral regions. Immunolabelled fibres were observed in most of the preoptic hypothalamus (Fig. 1G), including the medial preoptic, magnocellular preoptic, and preoptic periventricular nuclei. In the anterior hypothalamus, numerous CGRPi fibres were observed in the periventricular hypothalamic nucleus (Fig. 1H,I), including its magnocellular part, as well as in the lateral aspect of the anteromedial hypothalamic nucleus (not shown). In addition, a few immunolabelled fibres were present in the rostral lateral hypothalamic area.
In the tuberal hypothalamus (Fig. 1J–M), a relatively high density of immunolabelled fibres was present in the periventricular nucleus as well as surrounding the ventromedial hypothalamic nucleus (VMN) and its caudal continuation, the inferior nucleus of the hypothalamus (Kuenzel and Masson, 1988). Very few fibres were seen within the VMN itself. A few CGRPi fibres were present in the lateral hypothalamic area and in the median eminence, and a relatively dense CGRPi innervation was evident in both the medial and lateral mammillary nuclei.
Thalamus.
The most remarkable feature of the distribution of CGRPi fibres in the thalamus was their presence in the tractus ovoidalis, which appeared to arise from labelled neurones in the Ov-SPO and PMI and in the tractus thalamostriaticus, which appeared to arise in the DLP-DIP. These fibres ran rostralward and converged in the dorsal aspect of the FPL on their way to the telencephalon. In the rostral thalamus, the CGRPi fibres in the FPL developed a very dense terminal field in the nucleus reticularis superior pars dorsalis (RSd; Fig. 4A), where the presence of perisomatic figures suggested direct contacts with perikarya and proximal dendrites (Fig. 4B).
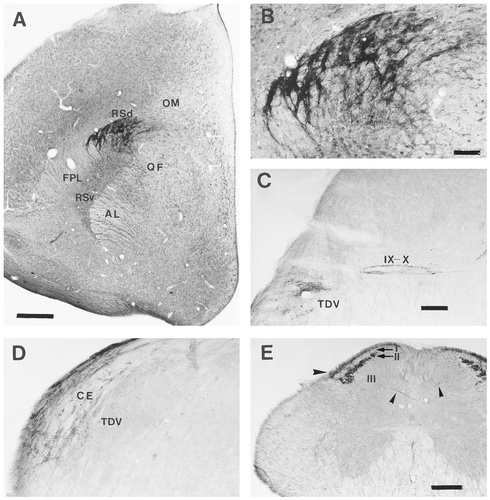
The extratelencephalic distribution of CGRPi terminal fields in the brain of the chick and quail. A: Low-power photomicrograph of a coronal section through the rostral diencephalon of a chick, showing the dense terminal field found in the dorsal part of the nucleus reticularis superior (Rsd). B: High-power photomicrograph showing CGRPi fibres in close apposition to perikarya and proximal dendrites of neurones in the Rsd. C: Photomicrograph of CGRPi fibres in the descending nucleus of the trigeminal nerve (TDV) of a quail at medullary levels, where immunoreactive fibres are also visible in the entrance of the glossopharyngeal and vagal nerves (IX-X). These fibres can be followed into the nucleus of the solitary tract (Fig. 1T).D: Photomicrograph of a CGRPi terminal field in the nucleus cuneatus externus (CE) of a quail, where descending fibres from the trigeminal nerve (TDV) and ascending ones from the spinal cord apparently converge. E: Low-power photomicrograph of a coronal section through the spinal cord of a quail, showing the dense CGRPi innervation of layers I (large arrowhead) and II of the dorsal horn, as well as scattered CGRPi fibres in deeper layers (small arrowheads). Labelled fibres, which probably belong to the TDV, are visible in the outer rim of the substantia gelatinosa. For abbreviations, see list. Scale bar = 50 μm in A, 100 μm in B,C (applies to B–D); 200 μm in E.
Sparse immunolabelled fibres were present in the dorsomedial anterior thalamic nucleus, the medial division of the dorsolateral anterior thalamic nucleus and in the periventricular thalamic nucleus. CGRPi fibres were also visible in the dorsomedial posterior thalamic nucleus (DMP), the medial aspect of the dorsolateral posterior thalamic nucleus (DLP), and in the nucleus dorsointermedius posterior thalami (DIP). Ventral to these nuclei, CGRPi fibres were located surrounding (but not within) the nucleus ovoidalis (Ov) and in the nucleus subrotundus (SRt) and PMI. It is interesting to note that the lateral aspect of the thalamus, including the nucleus rotundus and nucleus triangularis, the lateral aspect of the dorsolateral anterior thalamic nucleus (including the nucleus dorsalis intermedius ventralis anterior), the nucleus marginalis tractus optici, and nucleus geniculatus lateralis pars ventralis, were devoid of CGRPi fibres.
Pretectum and midbrain.
In the pretectum, a moderate number of CGRPi fibres was present in the area pretectalis. In addition, a few scattered immunolabelled fibres were observed around (but not within) the nucleus spiriformis medialis and the pretectal nucleus. CGRPi fibres from the pretectal area entered the periaqueductal grey and the tectal midbrain, where a sparse innervation of the optic tectum and intercollicular/toral region was observed. CGRPi fibres in the tectum were largely restricted to the subventricular part of the stratum griseum periventriculare, although a few fibres could be observed in the stratum griseum et fibrosum superficiale of the ventrolateral optic tectum. A greater density of immunolabelled fibres was present in the substantia grisea centralis, the nucleus of Darkschewitsch and the stratum cellulare externum. At this level, a few CGRPi fibres entered the oculomotor complex, where scattered immunolabelled fibres were observed dorsal and medial to the Edinger-Westphal nucleus and within the motor nuclei of the third nerve.
In the midbrain tegmentum, scattered immunolabelled fibres were present in the nucleus mesencephalicus profundus pars ventralis, in the mesencephalic reticular formation, and in the medial part of the substantia nigra (SN; Kitt and Brauth, 1986b), as well as medial to and occasionally within the red nucleus. The ventral tegmental area contained a moderate density of CGRPi fibres.
Brainstem and spinal cord.
Fibres displaying CGRP immunoreactivity were seen to course caudally from the periaqueductal grey into the isthmic/pontine region, where immunolabelled fibres were relatively dense in the parabrachial/coeruleal region (including the nucleus tegmenti ventralis). A few sparsely distributed fibres were also observed in the pontine reticular formation and subcoeruleal area. The immunolabelled fibres in the ventral tegmental area appeared to run caudally to enter the interpeduncular nucleus, where most terminated in either the ventral aspect of the interpeduncular nucleus or amongst the fibres of the descending brachium conjunctivum and in the nucleus of its decussation.
CGRPi fibres in the brainstem were most prominent in the sensory nuclei of the trigeminal, glossopharyngeal, and vagal nerves. A few varicose immunolabelled fibres were visible in the ventrolateral margin of the descending nucleus of the trigeminal nerve (Fig. 4C). These fibres apparently terminated in the nucleus cuneatus externus (Fig. 4D), where they appeared to intermingle with ascending fibres from the spinal cord (see below). In addition, a few varicose, labelled fibres were seen to enter the brain through the IXth and Xth nerves and to course medially (Fig. 4C) to enter the nucleus of the solitary tract (Fig. 1T).
The dorsal horn of the spinal cord contained many immunolabelled fibres forming a dense termination area in outer layers of the substantia gelatinosa (layers I and II; Fig. 4E). Scattered immunolabelled fibres were also present in deeper layers (III and IV) of the dorsal horn of the spinal cord. Sagittal sections clearly revealed CGRPi fibres to run within the dorsal columns. These fibres could be followed to medullary levels where dense termination fields were observed in the nuclei gracilis and cuneatus, as well as in the nucleus cuneatus externus (Fig. 4D). Some of the labelled fibres in the outer rim of the substantia gelatinosa may belong to the trigeminal system (Wild and Zeigler, 1996). Horizontal sections of the spinal cord showed CGRPi fibres running in what appeared to be the nucleus et tractus descendens nervi trigemini.
DISCUSSION
To our knowledge, the results of the current study of the domestic chick and Japanese quail provide the first comprehensive description of the distribution of CGRP-immunoreactive structures in the avian brain. The similarities between our results and those of previous (more restricted) studies of birds suggest that the CGRP antibody used in the current study recognises the same antigen previously immunolabelled in the pigeon (Brauth and Reiner, 1991; Berk et al., 1993) and zebra finch (Bottjer et al., 1997), and it shows virtually no cross-reactivity (<0.2%) with the closely related peptides calcitonin and calbindin (Sigma data sheets). The primary antibody was raised against rat CGRP type I (Amara et al., 1985), and although the amino acid sequence of CGRP is relatively well conserved, six of its amino acids differ from the published sequence of chicken CGRP (Minvielle et al., 1987). This difference may explain the need for the relatively high primary antibody concentration used in the current study. Immunolabelling of perikarya in the forebrain was uniformly dense, compared with the lighter and typically granular labelling in the midbrain and brainstem. The reason for this finding may be a lower concentration of CGRP in midbrain and brainstem perikarya compared with those in the forebrain. However, this difference in immunolabelling could also be caused by the presence of different species of CGRP in the chicken/quail, that have different affinities for the primary antibody used in the current study. The possibility that diverse species of CGRP occur in the central nervous system of birds, as is the case in rats and humans (Morris et al., 1984; Amara et al., 1985), requires further investigation.
Organization of CGRPi neuronal systems in birds
In the chick and quail, many CGRPi fibres were observed to enter the central nervous system in the Vth and IX-Xth cranial nerves, as well as in the dorsal roots of the spinal nerves. These CGRPi fibres developed terminal arborizations in primary sensory relay areas, e.g., the dorsal horn of the spinal cord, the gracile and cuneate nuclei, the nucleus of the solitary tract and the nucleus of the descending trigeminal tract, which also received descending trigeminal fibres. Thus, CGRPi is found within some quail and chick somatosensory neurones, as is the case in other vertebrates (Kawai et al., 1985; Kruger et al., 1988a,Kruger et al., 1988b; Petkó and Sánta, 1992; our unpublished observations of reptiles). Moreover, the only primary somatosensory perikarya that are located within the central nervous system, i.e., those constituting the mesencephalic trigeminal nucleus (Gottlieb et al., 1984; Byers et al., 1986), are CGRPi. However, the limited distribution of CGRPi fibres within primary somatosensory nuclei, indicates that only a small percentage of sensory ganglionic neurones are likely to be CGRPergic, as has been shown to be the case in mammalian dorsal root ganglia (Kruger et al., 1988b; Yang et al., 1998). Electrophysiologic evidence (Woodbury, 1992) indicates that the layers of the dorsal horn of the chicken spinal cord that are innervated by CGRPi fibres (layers I and II), respond mainly to noxious mechanical and thermal stimuli. This fact suggests that within the avian dorsal root ganglia, CGRP is contained in a specific subpopulation of sensory cells that is likely to be nociceptive and/or thermosensitive, as seems to be the case in mammals (Kruger et al., 1988b) and reptiles (our unpublished observations). Thus, the presence of CGRP in a specific population of sensory neurones seems to be a feature common to all vertebrates investigated. Because the trigeminal mesencephalic nucleus is known to be proprioceptive in mammals (Jerge, 1963), CGRP also appears to be expressed by some nonnociceptive sensory neurones. In view of the fact that sensory neurones presumably use excitatory neurotransmitters (Urban et al., 1994), it seems likely that CGRP coexists with glutamate/aspartate, as well as with other neuropeptides (Hökfelt et al., 1994).
A feature that chicks and quail share with other vertebrates is the presence of CGRP in motoneurons of the oculomotor, trigeminal, facial, and hypoglossal motor nuclei and in preganglionic parasympathetic neurones of the glossopharyngeal and vagal motor nuclei (Kawai et al., 1985; Kruger et al., 1988a,Kruger et al., 1988b; Petkó and Sánta, 1992; our unpublished observations of reptiles). Although the role of CGRP in somatic motor and preganglionic parasympathetic neurones is not clear, its presence indicates that CGRP probably coexists with acetylcholine. However, we have never observed CGRPi motor neurones in the ventral horn of the chick/quail spinal cord. This finding contrasts with the results of previous studies of mammals (Kawai et al., 1985; Kruger et al., 1988a) and other nonmammalian vertebrates (Petkó and Sánta, 1992; our unpublished observations of reptiles). Further research is needed to clarify whether this difference is attributable to technical limitations of the present study or actually reflects a functional specialisation of avian spinal motoneurons.
In addition to the CGRPi somatosensory and motor systems described above, there is evidence to suggest that CGRP is also present in several ascending projections from the thalamus, hypothalamus, and rostral pons. In the thalamus, CGRPi perikarya in the shell of the nucleus ovoidalis have been found in the pigeon (Brauth and Reiner, 1991) and zebra finch (Bottjer et al., 1997). To our knowledge, the current study is the first to demonstrate CGRPi perikarya in the DLP/DIP as well as in the PMI of the avian brain. Because all these CGRPi cell groups of the avian thalamus have been reported to project to the telencephalon (see references below), the possibility arises that immunoreactive perikarya in the thalamus are the source of at least part of the CGRPi innervation of the telencephalic hemispheres. Retrograde tracing experiments in pigeons have revealed ascending projections from the DLP and the Ov shell-SPO complex to different portions of the caudal (Waldman and Güntürkün, 1993; Metzger et al., 1996; Kröner and Güntürkün, 1999) and intermediate neostriatum and HV (Gamlin and Cohen, 1986; Wild, 1987b; Funke, 1989; Wild et al., 1993), the medial PA and PP (Veenman et al., 1997), and the caudomedial LPO (Wild, 1987a; Kröner and Güntürkün, 1999). The current study demonstrates that all of these telencephalic regions are rich in CGRPi fibres in the chick and quail.
The results of anterograde tracing experiments confirm the projections from the DLP and the Ov shell-SPO complex to the telencephalic areas, showing CGRPi innervation and in some cases reveal that the termination patterns of the thalamo-telencephalic projections match the distribution of CGRPi fibres. For example, tracer injections into the caudal DLP (see Fig. 7 in Gamlin and Cohen, 1986; see Fig. 3 in Wild, 1987b) result in labelling of a field of fine fibres and terminals that clearly approximates the dense CGRPi terminal field observed in the NI in the current study (see Figs. 1C–G, 3G,H). Moreover, Durand et al. (1992) traced the ascending projections from the shell of Ov in ring doves and demonstrated projections to the dorsal aspect of the HV (adjacent to the LFS), the caudal neostriatum (including a sparse projection to parts of the field L), the caudomedial paleostriatal complex (including the PVT), parts of the medial LPO, and to parts of the intermediate archistriatum and nucleus taeniae. This pattern of telencephalic projections closely resembles the distribution of CGRPi fibres in the telencephalon of the chick and quail.
It is interesting to note that, among the thalamic nuclei displaying CGRPi perikarya, only the Ov shell projects to auditory field L of the neostriatum (Durand et al., 1992). However, only a few scattered CGRPi fibres were observed in the field L of the chick/quail, whereas their density was greater in more lateral areas of the neostriatum (see results and Fig. 5). These findings contrast with those of Brauth and Reiner (1991), who reported a prominent CGRP innervation of field L in the pigeon. In contrast, Bottjer et al. (1997) did not find CGRPi fibre labelling in field L of the zebra finch.
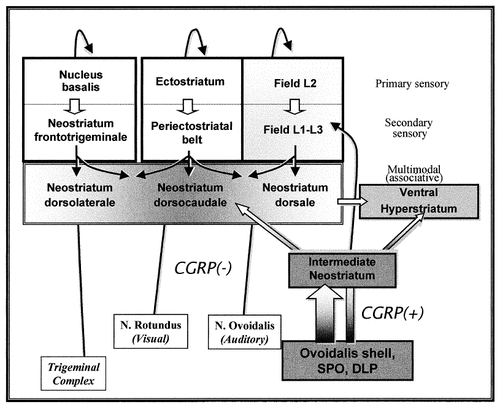
A diagram summarising the CGRPi innervation of the dorsal ventricular ridge of birds, in the context of the circuitry of the sensory telencephalon. The shading of the different areas of the hyperstriatum ventrale and neostriatum reflects the density of CGRPi fibres. It is assumed that these fibres arise mainly (if not exclusively) from the CGRPi neurones in the shell of nucleus ovoidalis, nucleus semilunaris paraovoidalis (SPO), and the nucleus dorsolateralis posterior thalami (DLP).
After large horseradish peroxidase injections into the NC, Funke (1989) reported retrograde labelling not only in the DLP and SPO, but also in the PMI, a cell group that contains CGRPi perikarya in chicks and quail. Furthermore, implantation of large deposits of lipophilic tracers in the septum of quail gave rise to retrogradely labelled perikarya in the ipsilateral PMI (Balthazart et al., 1994). Therefore, this midline nucleus is a potential source of the CGRPi innervation of both the caudolateral neostriatum and the septum.
Several nonthalamic cell groups displaying CGRPi neurones are known to project to the telencephalon and may contribute to the CGRPi innervation of the avian telencephalon. For instance, the nucleus of the ansa lenticularis has been reported to project to the septal area, the medial preoptic hypothalamus, or both, in quail (Balthazart et al., 1994). Similarly, two isthmic nuclei-displaying CGRPi perikarya, the locus coeruleus and the (putative) parabrachial nucleus, are known to give rise to ascending projections to the telencephalon (Kitt and Brauth, 1986a; Wild et al., 1990). Because some of the targets of these projections display CGRPi fibres, it is possible that the LoC, PB, or both, contribute to the CGRPi innervation of the telencephalon, as seems to be the case in mammals (Yasui et al., 1989, Yasui et al., 1991). However, the lack of CGRPi fibres in the tractus quintofrontalis (which is the pathway for the pontine projections to the telencephalon; Wild et al., 1990) argues against this possibility.
In addition to their telencephalic projections, thalamic and isthmic centres that contain CGRPi perikarya give rise to other ascending and descending projections, some of which might also be CGRPi. For example, the Ov shell apparently projects to the DLP/DIP complex, the ventral and dorsal hypothalamus, and the midbrain central grey (Durand et al., 1992). The latter also receives a projection from the PB, which extends to the subventricular part of the stratum griseum periventriculare (Wild et al., 1990), where CGRPi terminal fields were found in the current study of the chick and quail. Moreover, Wild (1987b) reported a projection from the caudal DLP to the RSd, which is richly innervated by CGRPi fibres in the chick and quail.
CGRP and the comparative neuroanatomy of the avian thalamus
Despite the important changes that the forebrain has undergone during the evolutionary history of vertebrates (e.g., Butler, 1994a,Butler, 1994b), CGRPi perikarya are found in the caudal aspect of the dorsal thalamus of all vertebrate groups that have been studied (mammals: Kawai et al., 1985; Kruger et al., 1988a,Kruger et al., 1988b; birds: Brauth and Reiner, 1991; Bottjer et al., 1997; reptiles: Brauth and Reiner, 1991; our unpublished observations in Podarcis hispanica; amphibians: Petkó and Sánta, 1992). A cladistic analysis of these data suggests that the CGRPi neurones in the thalamus of extant amphibians, reptiles, birds, and mammals are likely to be homologous.
In the rat forebrain, most of the CGRPi neurones form a single group in the posterior thalamus (Kawai et al., 1985; Kruger et al., 1988a,Kruger et al., 1988b; Yasui et al., 1989, Yasui et al., 1991), which extends from the central grey matter medially (some perikarya being found within the paraventricular nucleus, Kawai et al., 1985), to the peripeduncular nucleus laterally. This group of CGRPi cells includes the subparafascicular and posterior intralaminar nuclei, the posterior limitans thalamic nucleus (Price, 1995) and the suprageniculate nucleus (Skofitsch and Jacobowitz, 1985; Yasui et al., 1991). Despite the possibility that the rat CGRPi cell group in the posterior thalamus may have undergone expansion or reduction during evolution of the different lines of tetrapod vertebrates, it may be homologous with the thalamic CGRPi cell groups of reptiles and birds. In the caudal forebrain of birds, the most medial nucleus displaying CGRPi perikarya is the PMI, which may be comparable to the mammalian central grey just caudal to the central medial thalamic nucleus (Yasui et al., 1989). There have been few studies of the connections, cytoarchitecture, and/or chemoarchitecture of either the rostral central grey of mammals or the PMI of birds. However, this comparison fits the hypothesis put forward by Veenman et al. (1997) suggesting that the DMA/DMP (a thalamic cell group just rostral to the PMI) is the avian equivalent of the central medial thalamic nucleus of mammals (just rostral to the central grey in mammals). Moreover, the avian PMI (Balthazart et al., 1994) and the rat rostral central grey (see Beitz, 1995) share a projection to the septum and preoptic hypothalamus.
The CGRPi perikarya of the dorsal thalamus of birds (Ov shell, SPO, and DLP/DIP) are likely to be homologous with those of the subparafascicular/ posterior intralaminar thalamus of mammals. In addition to the presence of CGRPi neurones, these nuclei of the avian and mammalian thalamus also share a multimodal nature due to the convergence of a variety of sensory afferents. In birds, neurones in the shell of Ov and the SRt-SPO have been shown to respond to auditory stimuli (Durand et al., 1992), but they also receive a dense projection from the deep layers of the optic tectum (Gamlin and Cohen, 1986; Korzeniewska and Güntürkün, 1990), which may convey visual and somatosensory information (Cotter, 1976; Funke, 1989). Neurones in the shell of Ov and the SRt-SPO also constitute a target for the ascending projections of an area of the midbrain at the interface between the intercollicular nucleus and dorsal nucleus mesencephalicus lateralis (Durand et al., 1992), which has been shown to receive a massive projection from the dorsal column nuclei (Wild, 1989). Furthermore, the dorsal and medial parabrachial nuclei project to the paraovoidalis region in pigeons (Wild et al., 1990). The mammalian subparafascicular/posterior intralaminar thalamic complex receives auditory projections from the inferior colliculus, somatosensory (probably nociceptive) projections from the spinal cord (LeDoux et al., 1987), visceroceptive afferents from the parabrachial area (Saper and Loewy, 1980; Yasui et al., 1989), and both visual and nonvisual afferents from the deep layers of the superior colliculus (Yamasaki et al., 1986; Linke et al., 1999).
Veenman et al. (1997) have compared the avian DLP with part of the intralaminar thalamus of mammals (the caudal paracentral and central lateral nuclei), and the avian DIP with the parafascicular nucleus of mammals, on the basis of their location within the thalamus, their histochemical features and the connections with the basal ganglia. On the other hand, the DLP has been compared with the posterior complex of the mammalian thalamus, including the different subnuclei of the posterior nucleus and the suprageniculate nucleus (Gamlin and Cohen, 1986), on the basis of its multisensory nature (somatosensory, visual and auditory) and connections (Wild, 1987b; Korzeniewska and Güntürkün, 1990). The results of the current study suggest that the DLP/DIP is comparable to the posterior intralaminar thalamic complex of mammals, including the subparafascicular, lateral subparafascicular, suprageniculate and posterior intralaminar thalamic nuclei. Yasui et al. (1991) demonstrated that in the rat the projection from these posterior intralaminar thalamic nuclei to the dorsal striatum and central amygdala contains CGRP. The DLP of birds not only contains CGRPi perikarya, but also projects to parts of the avian equivalent of amygdala (see injections of retrograde tracers in the archistriatum by Kröner and Güntürkün, 1999) and the caudate-putamen (the medial aspect of the PA; Wild, 1987a,Wild, 1987b; Veenman et al., 1997), which both display CGRPi fibres. The latter projection also arises from the DIP, which also contains CGRPi perikarya. Taken as a whole, these hodological features and the presence of many CGRPi cells in the Ov shell, SPO and DLP/DIP of chick and quail, together with their position next to the main auditory relay of the dorsal thalamus (Ov), suggest that these CGRPi perikarya are comparable with the subparafascicular/posterior intralaminar nuclear complex of the mammalian thalamus.
CGRP and the comparative neuroanatomy of the avian telencephalon
Recently, Bottjer et al. (1997) reported the presence of a remarkable CGRPi cell group in the anterior neostriatum of male zebra finches. However, the absence of CGRPi perikarya from the telencephalon of chicks and quail in the current study and from pigeons (Brauth and Reiner, 1991), indicates that as a rule (song birds being an exception), the CGRPi innervation of the avian telencephalon has an extratelencephalic origin. Indirect evidence strongly suggests that the CGRPi fibres in the telencephalon of most birds arise from the thalamus, hypothalamus, and midbrain, as is the case in mammals (Shimada et al., 1985a,Shimada et al., 1985b; Yasui et al., 1989, Yasui et al., 1991) and reptiles (our unpublished observations). However, this possibility remains to be tested by using double-labelling techniques.
Three different CGRPergic projections to the telencephalon have been described in mammals. First a projection arising from the lateral hypothalamus and terminating in the lateral septum in the form of perisomatic baskets (Sakanaka et al., 1985). Similar CGRPi perisomatic baskets were found in the septum of birds in the current study and in reptiles (our unpublished observations). These similarities suggest that parts of the medial and lateral septum of birds are comparable to parts of the lateral (but not to the medial) septum of mammals, a similar situation to that found in reptiles (Font et al., 1995).
The second, well-characterised CGRPergic termination field of the mammalian telencephalon is also subcortical and comprises both the caudal basal ganglia and the amygdaloid complex (Kawai et al., 1985; Skofitsch and Jacobowitz, 1985). In fact, the densest CGRPi terminal field of the rat telencephalon lies at the boundary between the lateral division of the central amygdaloid nucleus and the fundus striatum (Kruger et al., 1988a,Kruger et al., 1988b), an area that has been referred to as the striato-amygdaloid transition area (Yasui et al., 1991). Rostral to this terminal field CGRPi fibres can be found to extend rostrally within the basal telencephalon as far as the caudal aspect of the nucleus accumbens (Kawai et al., 1985), giving rise to a sparse innervation of the “extended amygdala” (for review, see Alheid et al., 1995). A sparse innervation by CGRPi fibres also occurs next to the striato-amygdaloid transition area, in the lateral, basomedial (or accessory basal), medial, and cortical amygdala as well as in the caudal aspect of the caudate-putamen. Retrograde tracing after injections into either the fundus striatum and central amygdala or the caudate nucleus, gives rise to retrograde labelling of CGRPi cells in the posterior intralaminar thalamic cell group as well as in the parabrachial nucleus, the latter showing double labelling mainly after amygdaloid injections (Yasui et al., 1991).
The third CGRPi fibre system of the mammalian telencephalon terminates in the perirhinal and insular cortices, including the endopiriform nucleus and claustrum, (Kawai et al., 1985; Skofitsch and Jacobowitz, 1985) and arises from both the thalamic CGRPergic cell group and the parabrachial nucleus (Yasui et al., 1989). If the thalamic and parabrachial CGRPi cell groups of mammals and birds are homologous, then their targets in the telencephalon are also likely to be homologous. Therefore, the paleostriatum, LPO, and parts of the archistriatum, neostriatum, and hyperstriatum ventrale may be comparable to the striatum (caudate-putamen), extended amygdala, amygdala proper, and perirhinal and insular cortices (including the endopiriform nucleus and claustrum) of mammals.
Current ideas about the organisation of the basal ganglia of birds (e.g., Veenman et al., 1995; Medina and Reiner, 1997; Reiner et al., 1998) indicate that the avian paleostriatum augmentatum is comparable to the caudate/putamen and the paleostriatum primitivum is comparable to the globus pallidus. The presence of a CGRPi innervation of the caudal aspect of the paleostriatum, probably arising from the thalamic CGRPi cell group (and maybe parabrachial area; see discussion above) gives further support for this possible homology. However, the results of the current study reveal that the densest CGRPi termination field in the avian telencephalon is found in the paleostriatum ventrale (PVT). The PVT, as defined in the chick by Kuenzel and Masson (1987), appears to be a striatal territory belonging to the caudal edge of the LPO. Therefore, the PVT would include the avian counterpart of the striatoamygdaloid transition area as well as of the lateral division of the central amygdaloid nucleus of mammals. This suggestion is supported by both topographical (see above) and hodological data: for instance a projection from the parabrachial area reaches both the avian PVT (Kitt and Brauth, 1986a; Wild et al., 1990) and the central nucleus of the mammalian amygdala (Bernard et al., 1993; Alden et al., 1994).
Sparse CGRPi fibres can be followed rostrally from the dense terminal field in the avian PVT to an area of the basal ganglia that includes the bed nucleus of the stria terminalis and part of the caudal LPO. This innervation pattern is similar to the CGRP fibre system of the extended amygdala of mammals (Yasui et al., 1991). Therefore, the LPO and BNST would seem to include the avian homologue of the mammalian extended amygdala including its rostral end, the nucleus accumbens. This idea fits the current view of the avian basal ganglia/extended amygdala (see Veenman et al., 1995), as well as recent data on regional gene expression in the telencephalon of birds and mammals during development (Puelles et al., 1999), according to which the avian nucleus accumbens lies in the intermediate-to-rostral juxtaventricular LPO.
The presence of CGRPi fibres in the archistriatum supports the view that it includes at least part of the avian amygdala. The Am, which displays the densest CGRPi terminal field within the archistriatum, has been defined as the group of archistriatal neurones in close association with the fibres of the tractus occipito-mesencephalicus (Davies et al., 1997; Dubbeldam et al., 1997). Therefore, it is a good candidate for the intra-amygdaloid division of the bed nucleus of the stria terminalis as defined in mammals (Alheid et al., 1995). This is in agreement with recent gene expression data (Puelles et al., 1999), which suggest that the Am is the pallidal division of the archistriatum. The mammalian amygdala includes two pallidal structures, namely the medial amygdala and the intra-amygdaloid division of the BNST (Swanson and Petrovich, 1998; Puelles et al., 1999). The differential hodological feature of the medial amygdaloid nucleus of mammals is its massive input from the accessory olfactory bulb (Scalia and Winnans, 1975). Because most birds lack an accessory olfactory system (Reiner and Karten, 1985), a true medial amygdala may be absent in birds, leaving the only possible counterpart for the Am in the mammalian amygdala to be the BNST. However, whereas the majority of the neurones of the mammalian BNST are GABAergic (Price et al., 1987; Sun and Cassell, 1993), this situation seems not to be the case for the medial archistriatum of the pigeon (Veenman and Reiner, 1994).
In addition to the presence of CGRPi fibres in the paleostriatum and archistriatum, chicks and quail show a noteworthy CGRPi innervation of the caudal and intermediate neostriatum and the hyperstriatum ventrale (Fig. 5). From a comparative viewpoint, these findings suggest that the areas of the avian dorsal ventricular ridge displaying CGRPi innervation are comparable to parts of the mammalian insular/perirhinal cortex (including the claustrum) and basolateral division of the amygdala. This view fits the results of developmental studies that attribute a lateral pallial (Striedter and Beydler, 1997; Striedter et al., 1998) and ventral pallial origin (Puelles et al., 1999; intermediate area of Smith-Fernandez et al., 1998) to the avian dorsal ventricular ridge.
In view of the distribution of CGRPi fibres and of the available data on the connections of the avian telencephalon (Rehkamper and Zilles, 1991; Metzger et al., 1998; Kröner and Güntürkün, 1999), the different parts of the avian dorsal ventricular ridge may be divided in four different compartments (Fig. 5). First, there is a series of areas in the telencephalon that receives (apparently) unimodal thalamic projections of a visual (ectostriatum), trigeminal somatosensory (nucleus basalis), and auditory (field L2 of the neostriatum) nature and project to secondary sensory areas (ectostriatal belt, neostriatum fronto-trigeminalis, and neostriatal fields L1 and L3). Neither primary nor secondary sensory areas of the neostriatum receive an important CGRPi innervation (innervation of field L is scarce). Second, associative areas in the caudal neostriatum do receive an important CGRPi innervation, with the exception of the trigeminal associative neostriatum (neostriatum dorsolaterale according to Metzger et al., 1998). Third, our findings, together with hodological data from pigeons (Gamlin and Cohen, 1986; Wild, 1987a,Wild, 1987b) and chicken (Metzger et al., 1996, Metzger et al., 1998) suggest that the medial intermediate neostriatum is an associative area receiving multimodal thalamic CGRPi afferents from the DLP (visual, somatosensory, and auditory; Korzeniewska and Güntürkün, 1990) and is interconnected with the associative areas in the caudal neostriatum. Finally, the HV is a target for the secondary sensory and associative neostriatum and receives a prominent (probably thalamic) CGRPi innervation. Therefore, the CGRPi projection of the thalamus of birds seems to constitute a second multimodal input to the associative areas of the avian dorsal ventricular ridge. These data are compatible with both amygdaloid and claustral natures of the associative neostriatum, as suggested by the genetic data discussed above, but also indicate the presence of primary and secondary sensory areas in the avian neostriatum that have no clear counterpart in the claustro-amygdaloid complex of mammals.
In conclusion, the current data suggest that the avian amygdala might include not only parts of the archistriatum proper, but also structures rostrally adjacent to it within the basal telencephalon, which would constitute the avian counterpart of the mammalian extended amygdala. In addition, the associative neostriatum may be equivalent to parts of the basolateral division of the mammalian amygdala and also to the claustro-endopiriform complex. Detailed studies of the anatomy and histochemistry of the claustrum and endopiriform nuclei of mammals are required to clarify this issue.
Acknowledgements
We are indebted to Amparo Romero and Adoración Hernández for their help with the illustrations in this study, and to Alberto Segura from the farm “La Florida” for the gift of the day-old chicks used in this study.




