Lesions in the budgerigar vocal control nucleus NLc affect production, but not memory, of English words and natural vocalizations
Abstract
This study investigates the role of the psittacid (parrot) central nucleus of the lateral neostriatum (NLc) in the production of learned English and natural vocalizations. Anatomic data have led researchers to define NLc alternately as the parrot homologue (Paton et al. [1981] J Neurosci. 11:1279–1288) or analogue (Striedter [1994] J Comp Neurol. 343:35–56) of the songbird high vocal center. Although numerous functional and electrophysiological studies have identified the role of various songbird vocal control nuclei, few similar functional studies have been performed in parrots, particularly with respect to NLc. In this study, both novel behavioral techniques and precise neurochemical lesions have been used to investigate the role of NLc in the production of learned vocalizations. The results suggest that NLc is involved in the production of, but not memory for, learned English and natural vocalizations. Specifically, NLc lesions disrupted the amplitude of amplitude-modulated vocalizations, but did not affect the frequency of the dominant or carrier signal of these vocalizations. These data provide some of the first evidence for the functional role of a parrot vocal control nucleus. J. Comp. Neurol. 421:437–460, 2000. © 2000 Wiley-Liss, Inc.
Vocal learning, the ability to acquire vocalizations or acoustic features from an external model for later use in vocal production, is relatively rare in the animal world. Vocal learning has been identified in only humans, cetaceans, and birds (Kroodsma, 1982). Among birds, vocal learning occurs essentially in only 3 of the 28 orders: the Passeriformes (oscine songbirds), the Psittaciformes (parrots), and the Apodiformes (hummingbirds; Kroodsma, 1982; Kroodsma and Baylis, 1982; Gaunt et al., 1994). Avian vocal learning involves several stages. A bird must first perceive, decipher, and memorize (for at least some period of time) specific acoustic features of the target vocalization; i.e., create an auditory template. Spectral frequencies, amplitude, and the temporal pattern of a vocalization may, among other features, be critical for its proper replication and are, thus, incorporated as part of the template. To replicate the target vocalization, the bird must then convert the memorized auditory template to a set of instructions guiding motor production. Through repeated practice, the output of this “motor program” typically increases its resemblance to the initial auditory template and, thus, the target vocalization.
The neural bases of avian vocal learning have been studied in several songbirds and one parrot, the budgerigar (Melopsittacus undulatus); in both orders, a set of discrete and hierarchically arranged “nuclei” or cell groups functionally extend from the ear to syrinx (the sound source in birds), enabling these birds to engage in vocal learning (Nottebohm et al., 1976; Paton et al., 1981; Striedter, 1994; Durand, et al., 1997; see Brenowitz et al., 1997, for a review). In songbirds, lesion studies are commonly used to define the contribution of specific vocal control nuclei in the production of learned vocalizations (Nottebohm et al., 1976; Bottjer et al., 1984; Simpson and Vicario, 1990; Scharff and Nottebohm, 1991; Williams and Vicario, 1993). In contrast, few lesion or electrophysiological studies have investigated the functional role of psittacine vocal control nuclei (but see Hall et al., 1994; Heaton, 1997).
Parrots, because they exhibit an array of vocal plasticity, would seem to be appropriate subjects for such studies. Parrots are open-ended learners; therefore, like humans, and in contrast to many songbirds, their vocal learning is not constrained by age or season (Farabaugh et al., 1994). Parrots can mimic vocalizations of other species (Cruickshank et al., 1993) as well as the sounds of human speech (Pepperberg, 1981; Eda-Fujiwara and Okumura, 1992; Banta, 1998; Banta Lavenex, 1999). Furthermore, the Grey parrot (Psittacus erithacus), can use learned English vocalizations to solve tasks relating to a variety of complex cognitive constructs (e.g., referential labeling of color, shape, matter, and numerosity; Pepperberg, 1981; Pepperberg, 1990). Recently, budgerigars have also demonstrated some functional use of learned English vocalizations in their ability to produce primarily object labels, in contrast to other learned English vocalizations, when presented with target objects (Banta, 1998). Elucidating the functional role of the well-described nuclei and connections of the budgerigar vocal control system will, thus, provide important comparative evidence for understanding how the vertebrate brain organizes and executes behavior as complex as vocal learning.
This study was designed to investigate the role in vocal production of the budgerigar central nucleus of the lateral neostriatum (NLc, Fig. 1; the budgerigar anatomic nomenclature is from Striedter, 1994, and Durand et al., 1997). NLc is of interest because it receives input from a major auditory pathway through Bas and NF (by means of NLs; Striedter, 1994), regions that connect reciprocally with another auditory area, Field L (Farabaugh and Wild, 1997). NLc also projects directly to the archistriatal premotor nucleus, AAc (Paton et al., 1981; Striedter, 1994), which has direct connections with the hypoglossal nucleus (nXII) containing motor neurons innervating the tongue, trachea, and syrinx. In addition to elucidating the role of budgerigar NLc, these studies will also provide comparative evidence for assessing the role of the neostriatum in birds capable of learning vocalizations in general.
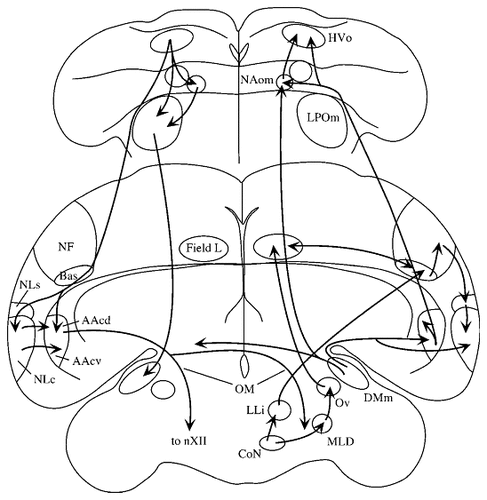
The budgerigar vocal control system. The major vocal control nuclei and their various connections are diagrammed. The figure shows two coronal sections, an anterior section (top) and a more posterior section at the level of NLc and AAc (bottom). For abbreviations, see list. Adapted from Durand et al., 1997, by permission of John Wiley & Sons, Inc.
Descriptions of the effects of NLc lesions on budgerigar contact calls or warble song are noticeably absent in the literature, which may be a consequence of one or a combination of factors: 1) syringeal and acoustic mechanisms used by budgerigars for vocal production of both warble song and contact calls were, until recently (Banta, 1998; Banta Lavenex, 1999; this study), poorly understood. Consequently, experimenters may have overlooked or misinterpreted postlesion changes in vocalizations in previous studies; 2) the complex and variable nature of budgerigar warble song (Farabaugh et al., 1992) may make prelesion and postlesion comparisons difficult; and 3) NLc is laterally adjacent to the archistriatal nucleus AAc to which it projects, and, thus, producing NLc lesions that do not affect the adjacent AAc is technically challenging. Accordingly, any investigation of the role of NLc in budgerigar vocal production requires 1) an alternative to studying the highly variable budgerigar warble song or poorly understood budgerigar contact call; 2) an understanding of the mechanisms of vocal production used by budgerigars; and 3) an extremely precise method of producing NLc lesions.
Previously, Banta and Pepperberg (1995) evaluated the feasibility of the use of English words and phrases acquired by budgerigars to study vocal learning and production. They found that budgerigars can be trained to produce specific words and phrases, which are then produced in two main behavioral contexts: 1) as elements enmeshed in their natural warble song (Eda-Fujiwara and Okumura, 1992; Banta and Pepperberg, 1995; Banta, 1998); and 2) as elicited vocalizations in response to presented objects (Banta and Pepperberg, 1995; Banta, 1998). Given these findings, trained English words seemed to represent a viable group of alternative “marker” vocalizations for studying the role of NLc in vocal production.
Preliminary analyses of prelesion vocalizations shed light on the poorly understood mechanisms of vocal production in the budgerigar. Of particular importance to the present study was the finding that budgerigar vowel sounds are not typical harmonic vocalizations as they are in humans and two other species of mimetic birds (mynahs, Gracula religiosa, Klatt and Stefanski, 1974; and Grey parrots, Patterson and Pepperberg, 1994). Their spectral features result instead from a nonlinear mechanism, amplitude modulation (Banta, 1998; Banta Lavenex, 1999). Budgerigars produce a 2,000–4,000 Hz carrier signal, then modify the amplitude of that signal with a modulating signal. In budgerigar productions of human vowel sounds, the carrier signal simulates the relative amplitude and time-variable frequency (although not the absolute frequency) of a human formant. The periodic modulating signal simulates the fundamental frequency of human speech. Furthermore, periodic, and likely aperiodic amplitude modulation, are key features of budgerigar contact calls (Banta, 1998; Banta Lavenex, 1999). This recent insight into budgerigar vocal production mechanisms may both facilitate detailed examination of the effects of lesions in NLc and help to elucidate how budgerigars produce these amplitude-modulated vocalizations.
Finally, NLc lesions were produced with an extremely precise method (see Materials and Methods section) intended to minimize the spread of damage to the medially adjacent premotor archistriatal nucleus AAc as well as other vocal control nuclei. The effects of the lesions described here are, thus, certain to result from disruption of the vocal control pathway at the level of NLc. The specificity of the lesions in this study should facilitate comparisons of the roles of different vocal control nuclei when such data become available.
In the present experiments, six male budgerigars were taught English words and phrases, then received lesions targeted at either NLc bilaterally (five birds), or, as a control, the dorsal and frontal neostriatum (NF; one bird). The five experimental animals received ibotenic acid lesions. Initial acoustic analyses targeted prelesion and postlesion English vocalizations. Data from these analyses guided assessment of the lesion effects on natural budgerigar contact calls. I describe the observed postlesion vocal deficits, compare prelesion and postlesion budgerigar words and contact calls with respect to several acoustic parameters, and identify parameters that are sensitive to the effects of NLc lesions. On the basis of my results, I propose roles for the budgerigar NLc and syrinx in vocal production.
Abbreviations
-
- AAa
-
anterior nucleus of the anterior archistriatum
-
- AAc
-
central nucleus of the anterior archistriatum
-
- AAcd
-
dorsal region of the central nucleus of the anterior archistriatum
-
- AAcv
-
ventral region of the central nucleus of the anterior archistriatum
-
- Bas
-
nucleus basalis
-
- CoN
-
cochlear nucleus
-
- DMm
-
magnocellular nucleus of the dorsomedial thalamus
-
- HA
-
accessory hyperstriatum
-
- HV
-
ventral hyperstriatum
-
- HVo
-
oval nucleus of the ventral hyperstriatum
-
- LH
-
hyperstriatal lamina
-
- LLi
-
intermediate lateral lemniscal nucleus
-
- LMD
-
dorsal medullary lamina
-
- LPOm
-
magnocellular nucleus of the parolfactory lobe
-
- MLD
-
laterodorsal mesencephalic nucleus
-
- N
-
neostriatum
-
- NAom
-
medial region of the oval nucleus of the anterior neostriatum
-
- NF
-
frontal neostriatum
-
- NFl
-
lateral region of the frontal neostriatum
-
- NLc
-
central nucleus of the lateral neostriatum
-
- NLs
-
supracentral nucleus of the lateral neostriatum
-
- NLv
-
ventral nucleus of the lateral neostriatum
-
- nXII
-
hypoglossal nucleus
-
- nXIIts
-
tracheosyringeal portion of the hypoglossal nucleus
-
- OM
-
occipitomesencephalic tract
-
- Ov
-
nucleus ovoidalis
MATERIALS AND METHODS
Animals
Subjects were six male budgerigars removed from a breeding aviary at fledging (4–5 weeks) and subsequently trained to produce human vocalizations. Two birds (Mosaic, Puck) were housed alone in sound-proof isolation boxes, with little auditory or visual contact with other birds; four birds (Forest, Grayson, Oliver, Peeper) were housed alone in cages, in auditory and visual contact with other budgerigars, Grey parrots, and many humans. Birds housed in social isolation had at least 1 hour of human interaction 5–6 days/week, and were exposed to auditory tapes (of either a human reading or soft classical and easy-listening music) for 6–8 hr/day. All birds received food and water ad libitum. Protocols used in this study were approved by the University of Arizona Animal Care and Use Committee.
Training of budgerigars
Budgerigars were trained to produce English words and phrases by using the Model/Rival (M/R) technique (Todt, 1975; Pepperberg, 1981) or a modified version (by using only one trainer; Banta and Pepperberg, 1995; Banta, 1998). M/R training involves two human trainers and one bird. The first human asks the second to label a particular item, e.g., a piece of paper. The second trainer responds with the appropriate label, “paper,” acting as both a model for the bird's targeted response and as the bird's rival for the attention of the first trainer. Trainers frequently exchange roles of questioner and respondent, eventually including the bird in the interaction. Budgerigars are rewarded for production of a correct object label, or an approximation of the label, by receiving the target object and vocal praise. After significant training with the M/R technique and two trainers, budgerigars were frequently trained by only one human that served both as a questioner and a model. Because by this time the birds were highly motivated to receive the target objects (toys that they enjoyed playing with), this technique was extremely effective to elicit the production of object labels.
Birds were trained for approximately 1 hr/day, 5–6 days/week, from approximately 6 weeks of age until lesioning (see Results section for specific ages). Target vocalizations were single words such as: “paper,” “cork,” “wood,” “bear,” and “truck.” Other vocalizations acquired by the birds were those used during training and social interactions such as “kiss,” “climb,” “tickle,” “you're right,” “okay,” “good boy,” “you be good,” and “come here.”
After lesioning, trainers conducted probe sessions. As in prelesion sessions, trainers would present and query the budgerigars about objects and reward elicited vocalizations with the object and vocal praise (“you're right,” “good boy”), but now would not produce or train object labels. Probe sessions allowed the birds' vocalizations to be recorded under similar behavioral conditions before and after lesioning, but did not give birds the opportunity to learn or re-learn object labels.
Audio recordings of vocalizations
Budgerigars were recorded at regular but long intervals (approximately every 45 days) before lesioning, and intensively (30–60 min/day, 4–6 days/week) for 3 weeks immediately before and after lesioning. Birds were recorded under two conditions: 1) during training (before lesions) and probe sessions (after lesions); and 2) while vocalizing freely on a perch or in their cage. All vocalizations used in this study were recorded when the birds were adult (at least 6 months old), and when the target vocalization was produced in a clear and stable manner. Vocalizations were recorded on Maxell XLII tapes with a Sony TCM 5000 voice-activated tape recorder and AKG C541 EB, Sennheiser ME 66 or ME 67 microphones.
Choice of vocalizations for acoustic analyses
Analyses focused on two different budgerigar vocalizations: vowels in English words and contact calls. Vowels were chosen because preliminary analyses revealed that abnormalities in NLc-lesioned budgerigar vocalizations were more noticeable in vowels than in consonants, probably because, in general, vowels or vowel sounds constitute most of the duration of most English syllables and words.
I determined which vowel sounds to analyze for each bird after extensively transcribing its prelesion training sessions, postlesion probe sessions, and prelesion and postlesion warble song bouts, and identifying each allospecific vocalization. Vocalizations that I could not unequivocally identify were classified “unidentifiable.” Thus, by the time postlesion transcriptions were completed, hundreds of words from each bird had been auditorally identified and rated with respect to quality and frequency of production. Because my analyses were restricted to vocalizations I could identify, I analyzed a subset of words that were produced an adequate number of times both prelesion and postlesion. All transcribed samples of each word (or sometimes a production type of a word, e.g., “paper” vs. “paaaperrrr”) were then analyzed according to objective measures so that any deficits would be quantitatively described, whether or not I could detect whether a specific exemplar was abnormal. I present vocalizations that are representative of the deficits exhibited by all birds in this study.
For all birds, contact call samples were randomly selected from single- and multiple-call productions. Because I could detect, auditorally, postlesion changes in calls of only one bird (Forest), I could not specifically chose contact calls that were abnormal for the other five birds. Every postlesion contact call for Forest was abnormal to my ear; his samples, thus, were also randomly selected from single- and multiple-call productions.
Acoustic analyses
Birds typically resumed prolific vocal behavior 12–24 hours after surgery. I present analyses of only those vocalizations produced at least 6 days after lesioning to ensure that the chemical lesions had produced their maximal damage, as well as to avoid ascribing nonspecific effects of surgical trauma (e.g., exacerbated effects caused by an injury response in the area) or ancillary effects of the chemical lesioning agent (e.g., excitotoxicity) to NLc lesions. Vocalizations were filtered <400 Hz and >10,000 Hz with a Hewlett Packard 8056A band pass filter and digitized with a Kay Elemetrics 5500 DSP Sona-graph (20,480 Hz sampling rate, 8 kHz frequency range). Spectral and amplitude waveform analyses were performed on the Kay and with SIGNAL (Beeman, 1996) sound analysis software.
I analyzed entire vowels, or 40-msec sections from relatively steady-state portions of the vowels, and entire contact calls. Figures show amplitude waveforms of entire words or syllables, as well as randomly selected excerpts (i.e., not necessarily the entire vowel or 40-msec section that was analyzed). The carrier and modulating signals of vowel sounds were calculated directly from the waveform of the 40-msec sections. As illustrated specifically in the Results section, the carrier signal was determined by taking the inverse of the average time between the peaks of the high frequency waveform oscillation, and the modulating signal determined by taking the inverse of the average time between the peaks of the low frequency waveform oscillation (Banta Lavenex, 1999). For contact calls, a 1,024-point power spectrum was used to identify the average dominant/carrier frequency of the call. Because the modulating signal of contact calls is extremely variable, and cannot be represented by any single average value, it was not measured. I used the “measure” function of SIGNAL to determine the maximal voltage (maxV) and root mean square (RMS; the average amplitude) of vocalizations or sections thereof.
The spectrograms and amplitude waveforms of prelesion and postlesion contact calls for each bird were cross-correlated to determine their similarity by using the “cormat” program in SIGNAL. Cross-correlation overlays two signals until their maximal overlap is achieved, and then computes a unitless index representing their degree of similarity (identical vocalizations = 1; vocalizations with no overlapping attributes = 0). Three different sets of cross-correlations were performed on each birds' prelesion and postlesion contact call samples: [pre × pre], [pre × post] and [post × post]. I then statistically compared correlation values for the following groups: [pre × pre] vs. [pre × post], [pre × post] vs. [post × post], and [pre × pre] vs. [post × post]. The similarity index presented in the Results section (Table 3) is an average of all correlation values for each group. A 1,024-point transform length (20 Hz analysis filter), with 100 transform steps, was used for spectrogram cross-correlations; spectrograms were not frequency-shifted or time-normalized (i.e., one signal expanded to match the length of the other). For waveform cross-correlations, signals were not time-normalized. Because the overlay technique gives more weight to comparing specific frequencies than to the overall shape of the vocalization, spectrogram cross-correlation analyses do not yield useful similarity scores for vocalizations that have complex spectra with many frequencies (e.g., given two nearly identical vocalizations, a difference in fundamental frequencies as small as 10-Hz translates into a 50-Hz difference in the 5th harmonic, a 100-Hz difference in the 10th harmonic, etc., thus, yielding a misleadingly low cross-correlation value). Thus, spectrogram and waveform cross-correlations were not performed on budgerigar word or vowel samples.
| Bird | Lesion method | Type of lesion | Volume2 of lesion (mm3) | % of NLc lesioned3 | % of Lesion in NLc4 | Other areas affected | |
|---|---|---|---|---|---|---|---|
| Type | Side | ||||||
| Forest | Ibotenic acid | Bilateral | Right | 0.899 | 49 | 75 | Ant N, NLs, NLv |
| Left | 0.551 | 34 | 75 | Ant N, NLs | |||
| Mosaic | Ibotenic acid | Bilateral | Right | 0.337 | 1 | 3 | Dorsal N |
| Left | 0.273 | 6 | 40 | NLs | |||
| Puck | Ibotenic acid | Bilateral | Right | 0.201 | 5 | 43 | Ant N, NLs |
| Left | 0.150 | 4 | 42 | Ant N, NLs | |||
| Grayson | Ibotenic acid | Bilateral | Right | 0.438 | 25 | 67 | Ant N, NLv |
| Left | 0.390 | 11 | 36 | NLv | |||
| Oliver | Ibotenic acid | Unilateral | — | — | — | — | — |
| Left | 0.255 | 1 | 8 | Ant N, NLs | |||
| Peeper | Electrolytic | Bilateral | Right | 0.560 | — | — | NF, NFl, N |
| Left | 0.505 | — | — | NF, NFl, N | |||
- 1 For abbreviations, see list.
- 2 The Cavalieri method was used to calculate the volume of each NLc nucleus, each lesion, and total volume of the lesion within NLc.
- 3 Percentage of NLc lesioned was calculated by dividing the volume of the lesion within NLc by the volume of NLc.
- 4 Percentage of lesion in NLc was calculated by dividing the volume of the lesion in NLc by the total volume of the lesion.
| Bird | Production2 | Max V | RMS | Max V:RMS | Abnormal amplitude fluctuations detected3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | P Value | Pre | Post | P Value | Pre | Post | P Value | |||
| Forest | |||||||||||
| /o/ | 1.996 ± 0.484 | 1.938 ± 1.549 | ns | 0.342 ± 0.104 | 0.239 ± 0.069 | <.02 | 6.050 ± 1.583 | 7.632 ± 3.689 | ns | Yes | |
| /e/ | 1.204 ± 0.266 | 1.765 ± 1.191 | ns | 0.372 ± 0.072 | 0.463 ± 0.297 | ns | 3.231 ± 0.303 | 3.786 ± 0.373 | <.0003 | Yes | |
| Contact calls | 2.197 ± 0.191 | 2.413 ± 1.077 | ns | 0.623 ± 0.128 | 0.470 ± 0.234 | <.03 | 3.639 ± 0.649 | 5.298 ± 1.089 | <.0001 | Yes | |
| Mosaic | |||||||||||
| /e/ | 2.112 ± 1.100 | 3.239 ± 2.271 | ns | 0.657 ± 0.320 | 0.910 ± 0.540 | ns | 3.203 ± 0.432 | 3.446 ± 0.622 | ns | Yes | |
| /e/ partial sample | 2.112 ± 1.100 | 5.406 ± 2.375 | <.0005 | 0.657 ± 0.320 | 1.440 ± 0.494 | <.0004 | 3.203 ± 0.432 | 3.690 ± 0.704 | ns | Yes | |
| Contact calls | 1.645 ± 0.719 | 2.718 ± 0.347 | <.0001 | 0.437 ± 0.137 | 0.571 ± 0.130 | <.004 | 3.685 ± 0.618 | 4.899 ± 0.764 | <.0001 | Yes | |
| Puck | |||||||||||
| /u/ | 3.082 ± 1.000 | 2.414 ± 1.090 | ns | 0.520 ± 0.141 | 0.446 ± 0.183 | ns | 5.911 ± 0.842 | 5.381 ± 0.722 | <.05 | Yes | |
| /u/ partial sample | 3.082 ± 1.000 | 1.422 ± 0.382 | <.002 | 0.520 ± 0.141 | 0.278 ± 0.090 | <.002 | 5.911 ± 0.842 | 5.212 ± 0.448 | ns | Yes | |
| Contact calls | 2.653 ± 0.431 | 2.211 ± 0.471 | <.004 | 0.692 ± 0.093 | 0.574 ± 0.106 | <.0006 | 3.850 ± 0.479 | 3.861 ± 0.430 | ns | ? | |
| Grayson | |||||||||||
| Contact calls | 2.595 ± 1.150 | 2.140 ± 0.578 | ns | 0.653 ± 0.198 | 0.538 ± 0.219 | ns | 3.868 ± 0.729 | 4.286 ± 0.907 | ns | Yes | |
| Oliver | |||||||||||
| Contact calls | 2.599 ± 1.124 | 3.496 ± 1.294 | <.03 | 0.642 ± 0.274 | 0.718 ± 0.242 | ns | 4.048 ± 0.559 | 4.842 ± 0.559 | <.0001 | ? | |
| Peeper | |||||||||||
| /er/ | 1.792 ± 0.944 | 2.525 ± 2.277 | ns | 0.297 ± 0.096 | 0.333 ± 0.213 | ns | 5.755 ± 1.253 | 6.752 ± 1.951 | ns | No | |
| /U/ | 1.620 ± 0.596 | 1.159 ± 0.528 | ns | 0.273 ± 0.078 | 0.174 ± 0.081 | <.03 | 5.827 ± 0.912 | 6.576 ± 1.066 | ns | No | |
| Contact calls | 2.379 ± 0.584 | 3.384 ± 0.597 | <.0001 | 0.557 ± 0.123 | 0.722 ± 0.119 | <.0001 | 4.278 ± 0.580 | 4.699 ± 0.429 | <.02 | No | |
- 1 ns, not significant.
- 2 Vowel sounds are /o/ from “okay”, the long “a” /e/ from “okay” and “paper”, the short “u” /u/ from truck, the “ear” /er/ from “bear”, and the “oo” /U/ from “wood”.
- 3 “Yes” or “No” responses were determinations made by the author after auditory and visual analyses of budgerigar vocalizations.
| Bird | Spectrogram XC | Waveform XC | Abnormal amplitude fluctuations detected | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A [pre × pre] | A:B | B [pre × post] | B:C | C [post × post] | Variability [pre vs post] A:C | A [pre × pre] | A:B | B [pre × post] | B:C | C [post × post] | Variability [pre vs post] A:C | ||
| Forest | 0.65 ± 0.10 | 0.52 ± 0.08 | 0.55 ± 0.08 | Increase | 0.21 ± 0.05 | 0.16 ± 0.04 | 0.17 ± 0.04 | Increase | Yes | ||||
| P<.0001 | P<.0001 | P<.0001 | P<.0001 | P<.001 | P<.0001 | ||||||||
| Mosaic | 0.60 ± 0.09 | 0.56 ± 0.07 | 0.58 ± 0.08 | – | 0.17 ± 0.07 | 0.17 ± 0.04 | 0.20 ± 0.04 | Decrease | Yes | ||||
| P<.0001 | P<.001 | ns | ns | P<0.0001 | P<.0001 | ||||||||
| Puck | 0.64 ± 0.07 | 0.57 ± 0.08 | 0.63 ± 0.07 | – | 0.25 ± 0.06 | 0.20 ± 0.05 | 0.23 ± 0.05 | Increase | ? | ||||
| P<.0001 | P<.0001 | ns | P<.0001 | P<.0001 | P<.0001 | ||||||||
| Grayson | 0.58 ± 0.12 | 0.52 ± 0.13 | 0.50 ± 0.17 | Increase | 0.19 ± 0.06 | 0.17 ± 0.06 | 0.16 ± 0.07 | Increase | Yes | ||||
| P<.0001 | P<.05 | P<.0001 | P<.0001 | ns | P<.0001 | ||||||||
| Oliver | 0.62 ± 0.10 | 0.57 ± 0.08 | 0.61 ± 0.09 | – | 0.20 ± 0.05 | 0.18 ± 0.04 | 0.20 ± 0.05 | — | ? | ||||
| P<.0001 | P<.0001 | ns | P<.001 | P<.0001 | ns | ||||||||
| Peeper | 0.54 ± 0.16 | 0.54 ± 0.12 | 0.58 ± 0.13 | Decrease | 0.20 ± 0.08 | 0.21 ± 0.08 | 0.24 ± 0.07 | Decrease | No | ||||
| ns | ns | P<.01 | ns | ns | P<.0001 | ||||||||
- 1 Means and standard deviations for spectrogram and amplitude waveform cross-correlation similarity indices are given for each bird for three different comparisons. pre, prelesion; post, postlesion. A:B = [pre × pre] vs [pre × post]; B:C = [pre × post] vs [post × post]; A:C = [pre × pre] vs [post × post].
Statistics
Means and standard deviations are given for quantitative measures. All statistical comparisons of prelesion and postlesion measures were made by using two-tailed t-tests for independent samples. For t-tests comparing the prelesion and postlesion spectrogram and waveform cross-correlations, tests were run on the raw cross-correlation values, and I used Levene's Test for Equality of Variances to determine the appropriate probability value for the comparison.
Lesions
Birds were anaesthetized with ketamine and xylazine (34 mg/kg and 17 mg/kg, respectively). After being anaesthetized, they received a 0.1-ml subcutaneous injection of 2% lidocaine under the scalp; 5 minutes later feathers were plucked from the scalp midline. The bird's head was positioned between the ear bars of a standard rat stereotaxic device, its scalp was opened with one sagittal incision, and the skull was positioned so that interaural zero was exactly 5 mm anterior to the bifurcation of the middorsal head sinus. A small screw was attached in an inverted position to the front of the skull with dental acrylic; this screw was used to immobilize the head so it could not pitch vertically.
Five experimental birds received ibotenic acid lesions. A 33-gauge Injector Cannula (Plastics One, Roanoke, VA) with a 25-cm-long piece of rubber tubing (Protech Intl., San Antonio, TX) attached to one end was filled with ibotenic acid (10 mg/ml; 7.7 pH; Sigma Chemical Co). A 10-μl syringe (Hamilton), also filled with ibotenic acid, was attached to the free end of the tubing, and was inserted into a microsyringe injection pump. The injection cannula was then positioned in a micromanipulator and advanced to previously determined stereotaxic coordinates in the budgerigar brain (Striedter, personal communication; Banta, personal observation). Once the cannula was in place, the injection pump infused 65–70 nl of ibotenic acid at a rate of 100 nl/min into the brain. Two minutes after infusion, the cannula was removed from the brain. The scalp was closed with Collodion (Fisher Chemical Co), and birds recovered from the anesthesia in a warm, humidified infant incubator. Birds were awake, eating, and vocalizing 2–4 hours after the surgery.
A sixth bird, the control subject (Peeper), received bilateral electrolytic lesions targeted at the lateral and central frontal neostriatum (NFl and NF). Control electrolytic lesions instead of ibotenic acid lesions are justified because electrolytic lesions, in theory, produce more extensive damage than ibotenic acid and, therefore, allow assessment of the nonspecific effects of surgery and brain lesion. For Peeper's lesion, all procedures were identical to those described above except that an epoxylite-coated insect pin and a stimulus isolation unit (70 μA of DC current for 55 msec), rather than an infusion of ibotenic acid, were used to make the lesions.
Histology
Three to 4 weeks after NLc lesions, and 9 months after the control NF/NFl lesion, birds received a lethal injection of ketamine and xylazine (102 mg/kg and 51 mg/kg, respectively) and were transcardially perfused with a 0.9% NaCl solution followed by 4% formal saline. Brains were removed, postfixed in 4% formal saline overnight, and then brought to equilibrium in a 30% sucrose solution for cryoprotection. Coronal sections (40 μm) were cut on a freezing/sliding microtome, mounted onto slides, and stained with cresyl violet.
Estimation of lesion volumes
The outline of the lesions and NLc nuclei for each bird were drawn at 80× magnification with the aid of a drawing tube. The Cavalieri method (Gundersen and Jensen, 1987) was used to calculate the volume of each NLc nucleus, each lesion, and total volume of the lesion within NLc. Because the anterior, posterior, and ventral borders of NLc are difficult to identify in Nissl-stained sections, I made every effort to be conservative when placing boundaries. The one exception to this rule was in the vicinity of the ventral-medial border of NLc, where the NLv protrudes between the NLc and the AAc (when NLc and AAc are at their maximal diameter). As this boundary was almost never visible, a portion of NLv was likely included in the estimation of NLc volume. Inclusion of this ventral-medial portion of the neostriatum in NLc volume measures surreptitiously inflates the NLc volume and, thus, decreases the estimate of the percentage of NLc lesioned.
RESULTS
General summary of lesions and effects
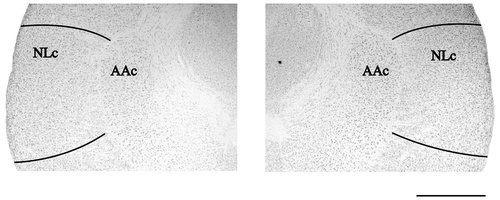
Five budgerigars received lesions targeted at NLc bilaterally, and one control subject (Peeper) received large bilateral control lesions targeted at the dorsal and frontal neostriatum (NF). Across the five experimental animals, variation existed in the amount of NLc lesioned, whether NLc was lesioned unilaterally or bilaterally, and whether the lesion was confined to NLc (Fig. 2A,B; Table 1, and below). In no NLc-lesioned subject was there evidence of damage in the medially adjacent archistriatal premotor nucleus, AAc, nor any other major vocal control nuclei (e.g., Bas, HVo, LPOm, NAom, or NFl).
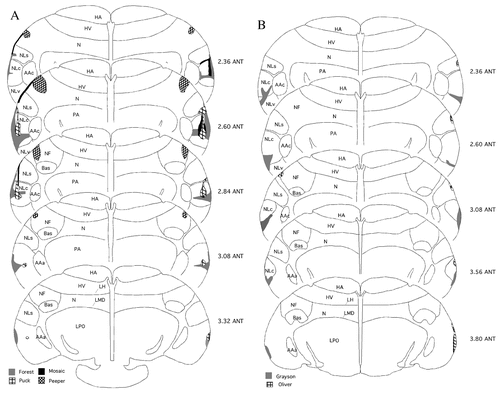
Lesion reconstructions. A: Reconstruction of lesions sustained by three experimental birds, Forest, Puck, and Mosaic, and the control bird, Peeper. B: Reconstruction of lesions sustained by two experimental birds, Grayson and Oliver. Lesion volumes are in Table 1. The section at the bottom is most anterior; the section at the top is most posterior. For abbreviations, see list. Sections are coronal, and spaced approximately 400 μm.
Four of five NLc-lesioned birds (Forest, Mosaic, Puck, and Grayson) produced vocalizations that I judged, auditorally, as abnormal immediately after recovery from surgery (24 hours after surgery; NB: only vocalizations produced at least 6 days after surgery are presented). Upon detailed inspection, the fifth NLc-lesioned bird, Oliver, proved to have disrupted vocalizations. The control-lesioned bird, Peeper, never produced any disrupted vocalizations. NLc lesions did not affect memory for any vocalizations in the birds' repertoire, their production rate (i.e., the relative number of times a given vocalization was produced), nor the overall temporal pattern or order in which word syllables and phrases were produced. Except for one bird (Grayson), abnormal postlesion behavior was confined exclusively to vocal disruptions, and no bird in this study (except Grayson) exhibited any other abnormal behavior, had observable problems breathing or eating, or exhibited abnormal posturing or head and beak movements when vocalizing. On four separate occasions, Grayson was observed having seizures lasting approximately 15–20 seconds, which seemed to cause temporary paralysis of the right side of his body. During seizures, Grayson's behavior, including all vocal activity, was arrested. Except for these four isolated incidents, Grayson's behavior seemed normal. The prelesion and postlesion vocal behavior of each bird is described below.
Forest
Description of lesions and general vocal behavior.
This 11-month-old budgerigar sustained the largest bilateral lesions, affecting a large extent of NLc, and spreading slightly into adjacent areas of the neostriatum (Figs. 2A, 3A,B; Table 1). The lesions spread to the border between NLc and the medially adjacent archistriatal premotor nucleus AAc, but did not spread into AAc. Forest's prelesion vocal repertoire included many English words and phrases produced primarily as elements enmeshed in warble song, and occasionally as elicited vocalizations. Nearly every postlesion production of both Forests' words and contact calls was obviously and severely disrupted; nonetheless, he warbled prolifically. Qualitatively, he sounded as if he was “gurgling” or talking underwater when he produced words and warble song.
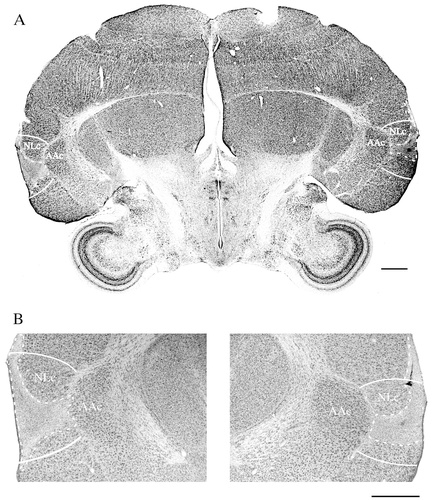
Photomicrograph of Forest's lesions. A: Low magnification image showing an entire coronal section at the level of NLc, and the largest medial/lateral extent of Forest's lesions (the right hemisphere is on the left). B: High magnification image of the section in A showing only the two lesions and surrounding brain regions. Neither lesion spread into the immediately adjacent AAc. For abbreviations, see list. Scale bars = 1,000 μm.
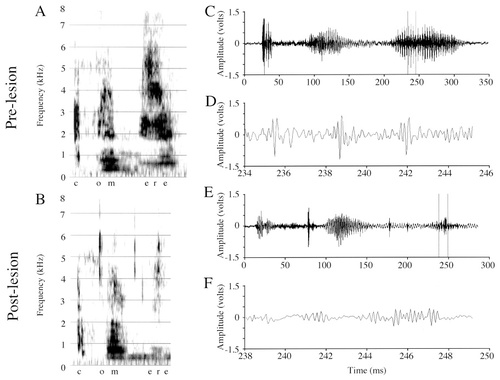
I present Forest's productions of the disyllabic “okay” to illustrate the effects of the lesions (Fig. 4; in the waveform figures, only the “kay” syllables and 10-msec excerpts from the “kay” syllables are shown; 40-msec sections of the /o/ and /e/ were analyzed). The nature and severity of the effects I present are representative of all words analyzed from Forest's repertoire. General inspection of wideband spectrograms of Forest's words (vowel sounds specifically; Fig. 4A,B) revealed frequent and highly abnormal amplitude fluctuations in nearly every postlesion word. Comparison of the amplitude waveforms of Forest's prelesion and postlesion vocalizations (compare the /e/ in Fig. 4C,D with E,F) revealed amplitude-modulated periods that were abnormally regular in appearance postlesion, and in which the alternating large- and small-amplitude regions of the carrier signal seemed to be more distinct and stereotyped than prelesion.
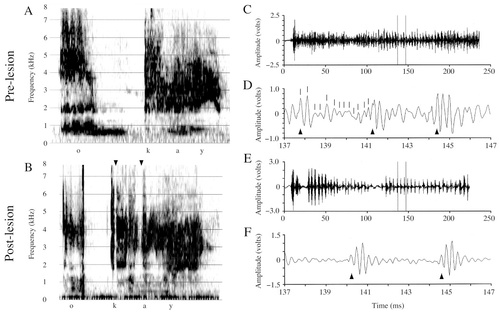
Spectrograms and amplitude waveforms of Forest's prelesion and postlesion productions of “okay.” For all vocalizations, A and B, respectively, present prelesion and postlesion wideband spectrograms (128 pts/234 Hz). For all spectrogram and waveform pairs presented, the vocalizations in the spectrograms are the same as those shown in the waveforms. For all waveforms presented, C and E are of the entire signal (either a word or a syllable of a word), and D and F are excerpts from the entire waveform demarcated by the vertical time cursors in C and E (time is given below the waveform of the excerpt). A: Prelesion production of “okay.” B: Postlesion production of “okay.” Note in the postlesion spectrogram the more greatly striated appearance and the apparent gaps in sound production (arrowheads). C: Amplitude waveform of the prelesion “kay” syllable from A. D: A 10-msec excerpt from C. The three arrowheads below the waveform in D demarcate the beginning of three periods of the modulating signal. Tick marks above the waveform demarcate the high frequency oscillations of the carrier signal. By calculating the inverse of the average time between these arrowheads or tick marks, one can determine, respectively, the modulating or carrier signal frequencies. E: Amplitude waveform of the postlesion “kay” syllable from B. Note that where apparent gaps in sound exist in the spectrogram (arrowheads in B), the high frequency signal is still visible in the waveform. F: A 10-msec excerpt from E. Note the large maximal voltage to average voltage ratio that is responsible for the striated appearance of the postlesion spectrogram (see Table 2).
Vowel sample analyses.
Carrier and modulating signal frequency.
Comparison of prelesion and postlesion samples of /o/ from “okay” revealed no postlesion differences in mean frequency of either the carrier signal (pre, 4,209 ± 419 Hz; post, 3,884 ± 934 Hz; t[18] = 0.917, P = 0.37) or the modulating signal (pre, 335 ±168 Hz; post, 299 ± 55 Hz; t[16] = 0.636, P = 0.54). The substantially smaller standard deviation for the postlesion modulating signal frequency may contribute to the stereotypic amplitude-modulated periods mentioned above. Comparison of prelesion and postlesion samples of /e/ from the “kay” syllable revealed that Forest's postlesion samples had a significantly faster carrier signal (see tick marks in Fig. 4D; pre, 3,294 ± 307 Hz; post, 3,895 ± 424 Hz; t[25] = −4.118, P = 0.0004), and a significantly slower modulating signal (see arrowheads in Fig. 4D,F; pre, 500 ± 123 Hz; post, 313 ± 107 Hz; t[24] = 4.011, P = 0.0005). Despite these frequency changes, distinct carrier and modulating signals are present throughout most, if not all, postlesion vocalizations.
Amplitude.
Forest's prelesion and postlesion vocalizations differed in amplitude. Table 2 presents raw values from two different measures (maxV and RMS) used to quantify amplitude characteristics, and their ratio (maxV:RMS), for all birds' vocalizations. The mean maxV of Forest's /o/ samples from “okay” (the entire vowel was analyzed) was not significantly different prelesion and postlesion (t[19] = 0.107, P = 0.92), signifying that, in general, Forest produced maximal amplitudes that were comparable before and after the lesion. In contrast, the mean RMS of Forest's /o/ samples was significantly smaller postlesion (t[19] = 2.752, P < 0.02), signifying that the average amplitude of these samples decreased postlesion. The maxV:RMS ratio (an indicator of how the maximal and average voltages vary relative to one another), although larger postlesion, was not significantly different from prelesion (t[19] = −1.20, P = 0.25), but note its very large variance. For /e/, I analyzed 40-msec sections taken between 110 and 190 msec in the syllable. Although neither the maxV (t[25] = −1.6, P = 0.13), nor the RMS (t[25] = −1.0, P = 0.32) was significantly different postlesion (Table 2), the maxV:RMS ratio was significantly greater postlesion (t[25] = −4.2, P = 0.0003).
Thus, for each slow period of Forest's vocalizations, the maximal peak-to-trough voltage observed postlesion is similar or slightly larger (although not significantly) than that produced prelesion. Postlesion, the average voltage (RMS) is either smaller than prelesion, or unchanged. In general, if postlesion maxV is unchanged or slightly increased, and postlesion RMS slightly decreased or unchanged, the maxV:RMS ratio will be greater for postlesion samples. The maxV:RMS ratio describes how, relatively, amplitude varies within the amplitude-modulated periods of a vocalization, and for Forest, the difference between the maximal and average voltages is greater postlesion. This seemingly minor difference creates a severe disruption in the perceived nature of “voicing” in Forest's vocalizations, even simulating a “temporal gap.” Thus, both auditorally and visually (in spectrograms), frequent and periodic bursts of sound alternate with apparent gaps in sound production (see arrowheads, Fig. 4B). However, inspection of the amplitude waveform confirms that sound is usually continuous and that bursts are not interspersed with “gaps” in sound production. Indeed, the carrier signal, because of its high frequency, can be observed “riding on top” of background noise even though it is at very low amplitude (see the portions of the waveform with very low amplitude in Fig. 4E).
Contact call analyses.
Inspection of wideband spectrograms revealed abnormalities in Forest's postlesion calls (Fig. 5B), but these differences were difficult to describe qualitatively. Time-alignment of contact calls with similar amplitude envelopes allowed direct observation of the effects of the lesions (Fig. 5C–F). The signal in the postlesion contact call (Fig. 5E,F) is not sustained in amplitude, and regions that should exhibit rapid amplitude fluctuations are drastically reduced in amplitude when compared with the prelesion call. These abnormalities are similar qualitatively to those seen in Forest's postlesion English vocalizations.
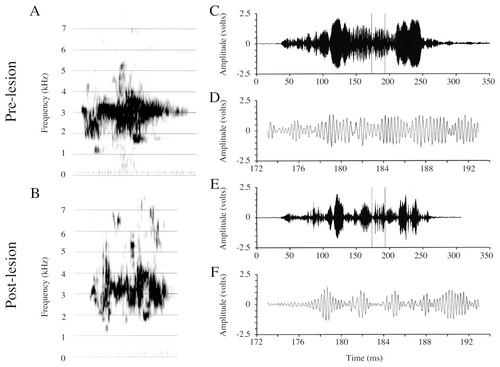
Spectrograms and amplitude waveforms of Forest's prelesion and postlesion contact call productions. A: Prelesion contact call. B: Postlesion contact call. C: Amplitude waveform of the prelesion contact call from A. D: A 20-msec excerpt from C. Note the smooth amplitude transitions throughout the waveform. E: Amplitude waveform of the postlesion call from B. F: A 20-msec excerpt from E. Note the sparse appearance of the entire signal waveform (E), and the abnormal modulation of the amplitude (F).
Frequency.
Budgerigar contact calls, although amplitude-modulated vocalizations, also exhibit changes in frequency with time. Figure 5A,B shows representative wideband spectrograms of Forest's prelesion and postlesion contact calls. To estimate the dominant (carrier) frequency present in each of Forest's calls, I identified the frequency at which peak amplitude occurred in a 1,024-point (20 Hz analysis filter) power spectrum of the entire call. No differences in the postlesion dominant frequency of Forest's calls were identified by this analysis (pre, 3,138 ± 655 Hz; post, 3,136 ± 777 Hz; t[34] = 0.01, P = 0.99).
Amplitude.
Voltage measures were taken over entire contact call samples. Two of three amplitude measures differed prelesion and postlesion (Table 2): the mean RMS was significantly smaller (t[34] = 2.28, P < 0.03), and the maxV:RMS ratio was significantly greater (t[34] = −5.25, P < 0.0001) postlesion. The average maxV was not significantly different (t[34] = −0.76, P = 0.44), but, as for the vowels, the variance of the postlesion samples was very large.
Spectrogram and amplitude waveform cross-correlations.
Both Forest's postlesion spectrograms and amplitude waveforms of contact calls were significantly less similar to the prelesion samples [pre × post] than were prelesion samples to themselves ([pre × pre]; Table 3; spectrogram cross-correlation t[387] = 16.76, P < 0.0001; amplitude waveform cross-correlation t[344] = 12.84, P < 0.0001). Although postlesion call spectrogram and waveform cross-correlations [post × post] had decreased correlation indices (i.e., increased variability) compared with prelesion cross-correlations [pre × pre] (for spectrograms, t[442] = 11.17, P < 0.0001; for waveforms, t[421] = 9.16, P< 0.0001), this increased variability could not account for all of the decrease in the [pre × post] correlation, because the [post × post] correlation index was significantly greater than the [pre × post] index (Table 3: for spectrograms, t[713] = −4.88, P < 0.0001; for waveforms, t[713] = −3.40, P = 0.001).
In summary, Forest's disruptions can be described as abnormal control of the fine amplitude modulations normally seen in amplitude-modulated vocalizations. Specifically, regions of both contact calls and English vocalizations that normally exhibit large peak amplitudes are increased in amplitude postlesion, and regions of vocalizations that normally exhibit small peak amplitudes are decreased in amplitude postlesion. This differential change in amplitude within amplitude-modulated periods of English vowels is responsible for the perceived temporal gap phenomenon, and the gurgly quality of Forest's vocalizations. Occasional disruptions in the frequency of the carrier and modulating signals in English vocalizations also occurred.
Mosaic
Description of lesions and general vocal behavior.
This 3.5-year-old budgerigar sustained a lesion in left NLc that spread slightly into the left NLs (see Table 1; Fig. 2A). The lesion attempt in the right hemisphere resulted in the spread of ibotenic acid up the cannula, with shallow but extensive damage to the neostriatal surface, and minor damage surrounding the cannula tract. In neither hemisphere did the lesions affect AAc. Mosaic's prelesion vocal repertoire consisted of a large number of English words and phrases produced both as elements enmeshed in warble song, and as elicited vocalizations. After surgery, vocal disruptions were obvious in many of Mosaic's word productions, but occurred sporadically rather than constantly as with Forest. Mosaic's vocal deficits occurred in both warble song and under elicited conditions; the deficits were similar in nature and frequency of occurrence across both categories (see below). I present his production of the word “paper,” which he produced prolifically both while warbling and under elicited conditions.
Vowel sample analyses.
Frequency.
No significant differences existed in the frequencies of either the carrier signal (pre, 4,097 ± 1,100 Hz; post, 4,257 ± 880 Hz; t[32] = −0.46, P = 0.64) or the modulating signal (pre, 356 ± 57 Hz; post, 371 ± 122 Hz; t[28] = −0.38, P = 0.69) in Mosaic's prelesion and postlesion /e/ samples from the “pa” syllable of “paper.”
Amplitude.
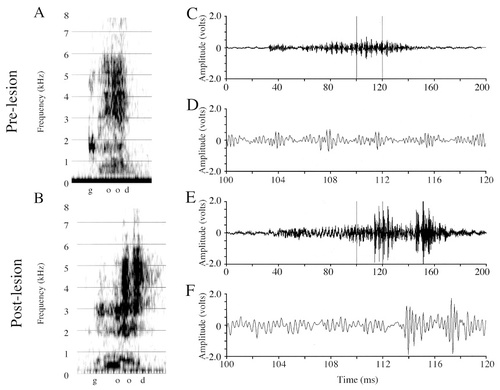
Figure 6B shows the spectrogram of one of Mosaic's postlesion “paper” productions exhibiting a large and abnormal amplitude increase (see arrow), and Figure 6E,F shows amplitude waveforms of the “pa” syllable. The abnormal amplitude increase occurs in just five amplitude-modulated periods of the vocalization, and these grossly exaggerated periods alternate with periods of normal, or smaller than normal, amplitude (see arrowheads in Fig. 6F). The carrier signal of this postlesion vocalization is amplitude-modulated at a rate of approximately 400 Hz. Within three amplitude-modulated periods, or ∼8 msec (from 36 to 44 msec), the maximal peak-to-trough amplitude of the carrier signal increases from 1.966 V to 9.427 V (a fivefold increase in the normal amplitude). Voltage analyses on 40-msec sections of Mosaic's prelesion and postlesion /e/ samples (Table 2) did not reveal any significant differences in the maxV (t[32] = −1.611, P = 0.11), RMS (t[32] = −1.48, P = 0.1486) or the maxV:RMS ratio (t[32] = −1.201, P = 0.23). Amplitude differences were likely not significantly different because only a subset of postlesion /e/ samples was disrupted. When only those postlesion samples identified as containing amplitude increases were compared with prelesion vocalizations, (Table 2, /e/ partial sample) however, both maxV (t[18] = −4.21, P = 0.0005) and RMS (t[18] = −4.32, P = 0.0004) were significantly increased. The maxV/RMS ratio differences between these prelesion and postlesion samples just failed to reach significance (t[18] = −1.93, P < 0.07).
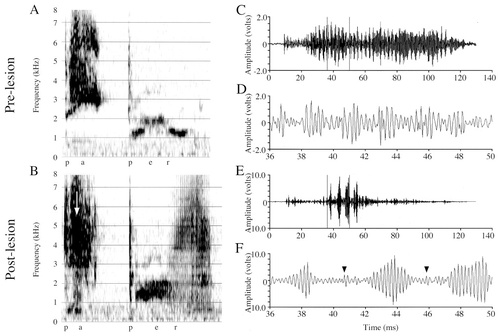
Spectrograms and amplitude waveforms of Mosaic's prelesion and postlesion productions of “paper.” A: Prelesion production of “paper.” B: Postlesion production of “paper.” Note in the postlesion spectrograms the very dark, high-amplitude region in the /e/ syllable of “pa” (white arrow), and the abnormal broadband appearance of the “per” syllable. C: Amplitude waveform of the entire prelesion “pa” syllable from A. D: A 14-msec excerpt from C. E: Amplitude waveform of the entire postlesion “pa” syllable from B. F: A 14-msec excerpt from E. The postlesion signal exhibits highly abnormal increases in the amplitude of just four amplitude-modulated periods. Note the difference in the amplitude scales between C,D and E,F. Arrows between the large amplitude increases in F denote the beginning of amplitude modulated periods that are either of normal, or slightly decreased, amplitude. The postlesion signal also is not completed at normal amplitude (i.e., amplitude fails before the end of the vocalization).
As mentioned above, Mosaic produced “paper” both under elicited conditions and while warbling. Of the postlesion elicited “paper” productions, 12 of 22 (54.8%) were categorized auditorally and visually (in spectrograms and waveforms), as having abnormal amplitude fluctuations (8 with amplitude increases, and 6 with amplitude failures [described below for the subject, Puck]; NB: some vocalizations contained both increases and failures at different points). For warbled “paper” productions, 10 of 18 (55.6%) had abnormal amplitude fluctuations (4 with amplitude increases, 7 with amplitude failures).
Contact call analyses.
Although I could hear no obvious disruptions in Mosaic's postlesion calls, visual inspection and time-alignment of calls with similar amplitude envelopes allowed me to identify abnormalities similar to, but less frequent than, those seen in Forest's contact calls (Fig. 7C–F). The postlesion call shown exhibits a significant amplitude failure (between time cursors in Figs. 7E,F), resulting in a drastic amplitude decrease that lasts 4 msec, rather than 1 msec as in the normal prelesion call. The postlesion call eventually recovers from this amplitude failure, and then is terminated in a relatively normal manner.
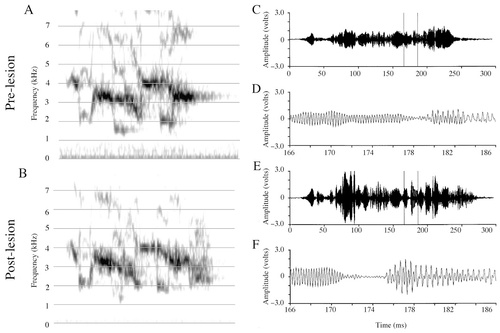
Spectrograms and amplitude waveforms of Mosaic's prelesion and postlesion contact call productions. A: Prelesion contact call. B: Postlesion contact call. C: Amplitude waveform of the prelesion contact call from A. D: A 20-msec excerpt from C. E: Amplitude waveform of the postlesion call from B. F: A 20-msec excerpt from E. Note the abnormally small amplitude of the waveform and the extended time course of the amplitude decrease (F).
Spectrogram cross-correlations (Table 3) revealed that the [pre × post] comparison group was significantly less similar than the [pre × pre] group (t[521] = 6.77, P < 0.0001), and call variability was not significantly different postlesion compared with prelesion ([pre × pre] vs. [post × post], t[394] = 1.91, P = 0.057). Amplitude waveform cross-correlations did not reveal any differences between the prelesion and postlesion comparison groups ([pre × pre] vs. [pre × post]; t[390] = −0.31, P = 0.76), but postlesion call waveforms were significantly less variable than prelesion ([pre × pre] vs. [post × post]; t[394] = −3.57, P < 0.0001; [pre × post] vs. [post × post]; t[173] = −5.08, P < 0.0001), indicating that the call amplitude waveform was produced in a more stereotypic manner postlesion. Voltage measures of entire contact call samples (Table 2) revealed that postlesion measures were significantly greater in maxV (t[38] = −5.53, P < 0.0001), RMS (t[38] = −3.07, P < 0.004), and the maxV:RMS ratio (t[38] = −5.53, P < 0.0001).
Mosaic's disruptions can be summarized as severe and disruptive amplitude fluctuations, including (1) large, abnormal increases in the amplitude of the carrier signal, modulating signal, or both as shown above; and (2) large, abnormal decreases in (or failures to increase) the amplitude of the carrier signal, modulating signal, or both, otherwise termed “amplitude failures.” A detailed acoustic analysis of amplitude failures is not presented for Mosaic, but is presented for Puck.
Puck
Description of lesions and general vocal behavior.
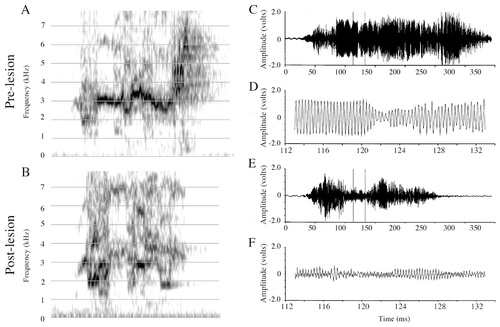
This 3-year-old budgerigar sustained small bilateral NLc lesions that spread slightly into the anterior neostriatum and the overlying NLs, but that did not affect AAc at any level (Table 1; Fig. 2A). Puck's prelesion vocal repertoire consisted of a large number of English words and phrases produced both as elements enmeshed in warble song and as elicited vocalizations. Puck's postlesion vocalizations exhibited intermittent disruptions similar to those observed in Mosaic. The disruptions of Puck's English vocalizations under elicited conditions and in warble song were similar in nature and frequency of occurrence. I present his prelesion and postlesion productions of the word “truck” (Fig. 8).
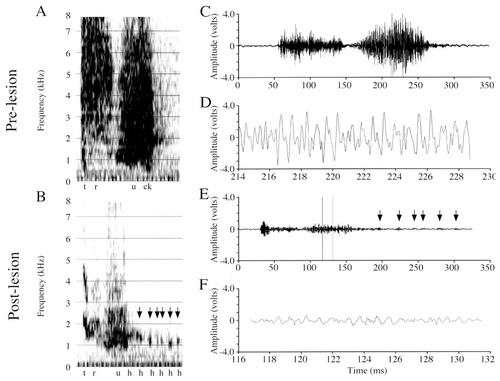
Spectrograms and amplitude waveforms of Puck's prelesion and postlesion productions of “truck.” A: Prelesion production of “truck.” B: Postlesion production of “truck.” C: Amplitude waveform of the entire prelesion “truck” syllable from A. D: A 14.7-msec excerpt from C. E: Amplitude waveform of the entire postlesion “truck” syllable from B. F: A 14.7-msec excerpt from E. The postlesion signal exhibits a highly abnormal decrease in the amplitude, both in the “tr” and “u” regions. Note, however, the small, high-frequency bursts in the postlesion signal (arrows in B and E) that extend well beyond the duration of the prelesion signal (e.g., compare the length of signal in C [238 msec] with that in E [277 msec]).
Vowel Sample Analyses.
Amplitude.
In the postlesion “truck” waveform (Fig. 8E,F), the carrier signal in the /u/ vowel never increases to its normal size (compare with Fig. 8C,D) and eventually subsides (Fig. 8E), hence receiving its designation as a vocal failure. However, the carrier signal does return sporadically throughout the remaining duration of the vocalization (see arrowheads in Fig. 8B,E), in this case for a period of time longer than normal. To the ear, this vocalization sounds like “truh-h-h.” When the carrier signal returns, although at a frequency comparable to prelesion, it is significantly diminished in amplitude and never recovers its regular cyclic amplitude fluctuations. Voltage measures taken on entire “truck” samples (Table 2) revealed that the maxV:RMS ratio was significantly smaller postlesion (t[37] = 2.11, P < 0.05), and the difference between the prelesion and postlesion maxV just failed to reach significance (t[37] = 1.99, P = 0.05; with postlesion maxV smaller). The RMS (t[37] = 1.412, P = 0.16) was not significantly different prelesion and postlesion. When only those postlesion samples identified as “amplitude failures” were compared with prelesion vocalizations (Table 2, /u/ partial sample), both maxV (t[22] = 3.59, P < 0.002) and RMS (t[22] = 3.62, P < 0.002) decreased significantly. The maxV/RMS ratio did not vary between these prelesion and postlesion samples (t[22] = 1.77, P = 0.09).
Of 20 postlesion “truck” productions analyzed from warble and elicited conditions, at least seven productions were disrupted, nine productions did not appear to be disrupted either auditorally or visually (in spectrograms and waveforms), and four productions could not be definitively identified as disrupted or not. Five of the seven disrupted productions were what I describe as “amplitude failures.”
Contact call analyses.
Auditorally, there were no obvious disruptions in Puck's postlesion contact calls, and visual inspection of amplitude envelopes did not reveal any definitive abnormalities in postlesion calls. However, spectrogram and amplitude waveform cross-correlations (Table 3) revealed that the [pre × post] comparison group was significantly less similar than the [pre × pre] group (spectrogram correlation t[588] = 10.56, P < 0.0001; amplitude waveform correlation t[300] = 8.85, P < 0.0001). Increased variance in the postlesion vocalizations could not account for the difference in the correlation values for spectrograms ([pre × pre] vs. [post × post], t[378] = 1.18, P = 0.24). For waveforms, although the postlesion variance was significantly increased compared with prelesion ([pre × pre] vs. [post × post], t[363] = 3.89, P < 0.0001), this increased variance could not account for all of the change in the [pre × post] correlation values as the [pre × post] group was significantly less similar than the [post × post] group, t[588] = −5.25, P < 0.0001. Voltage measures taken on entire contact call samples revealed that postlesion calls had significantly smaller maxV (t[38] = 3.09, P < 0.004), and RMS values (t[38] = 3.74, P = 0.0006), but the postlesion maxV:RMS ratio did not differ from the prelesion value (t[38] = −0.07, P = 0.93; Table 2).
Puck, like Mosaic, exhibited two main types of vocal deficits: (1) large, abnormal increases in the amplitude of the carrier signal, modulating signal, or both; and (2) decreases in the amplitude of the carrier signal, modulating signal, or both. The amplitude increases were similar in nature and severity to those described for Mosaic and, thus, are not described for Puck. When amplitude failures were observed, there was a failure of the carrier signal to increase in a periodic manner to its normal maximal amplitude.
Grayson and Oliver
Description of lesions and general vocal behavior.
These two birds, 14 months and 2 years, respectively, both sustained damage to NLc and the surrounding neostriatum (Table 1; Fig. 2B). Although Grayson's lesions affected a large portion of NLc, note that a significant portion of both lesions fell on the ventral border of NLc. For Oliver, only his left hemisphere sustained damage. AAc was not damaged in either bird. Grayson's prelesion vocal repertoire consisted of numerous words and phrases that he produced both enmeshed in warble song and as elicited vocalizations. Auditory and visual analyses identified significant disruptions in his postlesion English vocalizations. Oliver's prelesion vocal repertoire consisted of a large number of words and phrases that he produced with extreme clarity, but that were enmeshed as elements in warble song exclusively; he never produced elicited vocalizations. Although the effects of Oliver's lesion were minor, extensive word-by-word analyses revealed that some of his vocalizations exhibited disruptions. Because the effects of lesions on Grayson's and Oliver's vocalizations were variably intermittent (and minor, for Oliver), quantitative amplitude analyses did not represent their disruptions in terms of global prelesion and postlesion differences. Thus, I present spectrograms and waveforms from one prelesion and postlesion vocalization from each bird that are representative of their vocal disruptions.
Vowel sample analyses.
Grayson's postlesion production of the phrase “come here” (pronounced “comere;” Fig. 9A–F) exhibits both carrier signal amplitude increases (at ∼80 and 180 msec) and amplitude failures (from ∼200 to 300 msec). Oliver's production of the word “good” (Fig. 10A–F) exhibits two abnormal increases in carrier signal amplitude (at ∼114 and 145 msec).
Spectrograms and amplitude waveforms of Grayson's prelesion and postlesion productions of “come here.” A: A prelesion production of “come here” (pronounced “comere”). B: A postlesion production of “come here.” Note in the postlesion spectrograms the high-frequency amplitude burst in the vicinity of the “o,” and the nearly complete failure of the vocalization in the “here” syllable. C: Amplitude waveform of the entire prelesion “come here” vocalization from A. D: An 11.2-msec excerpt from C. E: Amplitude waveform of the entire postlesion “come here” vocalization from B. F: An 11.2-msec excerpt from E. Note the highly abnormal high frequency burst at approximately 75 msec (the burst in the vicinity of the “o” in the spectrograms) and the failure of the high-frequency signal after 175 msec. Note also the abnormal high frequency bursts around 180 msec and 200 msec that are also visible in the spectrograms.
Spectrograms and amplitude waveforms of Oliver's prelesion and postlesion productions of “good.” A: A prelesion production of “good.” B: A postlesion production of “good.” Note in the postlesion spectrograms the two high-amplitude bursts in the vicinity of the /U/. C: Amplitude waveform of the entire prelesion “good” vocalization from A. D: A 20-msec excerpt from C. E: Amplitude waveform of the entire postlesion “good” vocalization from B. F: A 20-msec excerpt from E. Note the highly abnormal high frequency bursts at approximately 114 msec and 145 msec. The excerpt in F shows the evolution of the first high-frequency burst, how it is preceded by poorly defined amplitude-modulated periods, and then what appears to be a decrease in the signal amplitude.
Contact call analyses.
Auditorally, there were no disruptions in either Grayson's or Oliver's postlesion contact calls, but visual inspection of the amplitude waveforms revealed abnormalities in some amplitude envelopes of Grayson's calls. I present one contact call from Grayson that contains severe amplitude fluctuations, specifically failures, from which the call does not recover (Fig. 11A–F). Note in the expanded waveforms (Fig. 11D,F) how the postlesion call is never able to increase to a normal, sustained amplitude. Although at one point, the call does begin to increase in amplitude (∼140 msec), this increase is not sustained, and the call eventually ends prematurely.
Spectrograms and amplitude waveforms of Grayson's prelesion and postlesion contact call productions. A: Prelesion contact call. B: Postlesion contact call. C: Amplitude waveform of the prelesion contact call from A. D: A 20-msec excerpt from C. E: Amplitude waveform of the postlesion call from B. F: A 20-msec excerpt from E. Note the abnormally small amplitude of the envelope (E) and the abnormal appearance of the waveform (F).
Spectrogram and amplitude waveform cross-correlations revealed that for both birds the [pre × post] comparison group was significantly less similar than the [pre × pre] group (Table 3). For Grayson, spectrogram correlations are t[588] = 5.43 and P < 0.0001, and amplitude waveform correlations are t[588] = 4.90 and P < 0.0001. For Oliver, spectrogram correlations are t[306] = 6.53 and P < 0.0001, and amplitude waveform correlations are t[317] = 3.28 and P = 0.001. For Grayson, increases in both spectrogram and waveform variability postlesion could account for the increased variability in the [pre × post] comparison group ([pre × pre] vs. [post × post], for spectrograms, t[341] = 4.83, P < 0.0001; for waveforms, t[372] = 4.16, P = 0.0001; [pre × post] vs. [post × post], for spectrograms, t[293] = 0.94, P = 0.35; for waveforms, t[299] = 0.65, P = 0.52). For Oliver, postlesion spectrograms and waveforms were not increased in variance compared with prelesion and, thus, could not account for the changes in the [pre × post] correlation values ([pre × pre] vs. [post × post], for spectrograms, t[378] = 1.31, P = 0.19; for waveforms, t[378] = −0.39, P = 0.69; [pre × post] vs. [post × post], for spectrograms, t[333] = −5.39, P < 0.0001; for waveforms, t[588] = −4.17, P < 0.0001).
Voltage measures of entire contact call samples from Grayson (Table 2) showed no significant prelesion and postlesion differences for any of the three measures (maxV t[38] = 1.584, P = 0.12; RMS t[38] = 1.75, P = 0.08; maxV:RMS ratio t[38] = −1.60, P = 0.11). Voltage measures of entire contact call samples from Oliver (Table 2) showed that postlesion contact calls had a larger maxV (t[38] = −2.34, P < 0.03) and a larger maxV:RMS ratio (t[38] = −4.49, P < 0.0001) but that RMS was not different (t[38] = −0.92, P = 0.36) from prelesion values.
In summary, both Grayson and Oliver exhibited vocal disruptions that, although intermittent, were qualitatively similar to the postlesion disruptions exhibited by the other birds. Grayson's postlesion “come here” illustrates the presence of both rapid and abnormal increases in the amplitude of the carrier signal, modulating signal, or both, and carrier signal amplitude failures, modulating signal amplitude failures, or both. Grayson also occasionally produced vocalizations that exhibited impairments in fine amplitude control (similar to Forest's vocalizations; not shown). All of Oliver's disrupted vocalizations exhibited either rapid and abnormal increases in the amplitude of the carrier signal, modulating signal, or both (as illustrated with his “good” production) or failures of the carrier signal amplitude, modulating signal amplitude, or both (not shown).
Peeper
Description of lesions and general vocal behavior.
This 2.5-year-old male budgerigar sustained large bilateral, electrolytic lesions in the dorsal neostriatum (Table 1; Fig. 2A). Because the plane of section may be misleading as to the absolute anterior/posterior position of Peeper's lesion (and only experiments to determine where the cells in this area project could provide definitive evidence), it is difficult to conclude whether some of Peeper's lesion affected the dorsal frontal neostriatum proper (NF and NFl), or only the dorsal central neostriatum (N). Nevertheless, neither nucleus NLc nor AAc was affected by the lesions (Fig. 12). Peeper's prelesion repertoire consisted of English words and phrases enmeshed in warble song; he did not produce elicited vocalizations. Postlesion abnormalities could not be detected in any of the hundreds of Peeper's words that I analyzed auditorally and visually.
Photomicrographs of Peeper's unlesioned NLc. High-magnification images showing the two NLc nuclei at their largest medial/lateral extent (the right hemisphere is on the left). Note that neither NLc shows any signs of a lesion. For abbreviations, see list. Scale bar = 1,000 μm.
Vowel sample analyses.
Prelesion and postlesion amplitude measures from Peeper's productions of “bear” and “wood” (Table 2) and spectrograms and amplitude waveforms from his productions of “wood” (Fig. 13A–F) are presented. Note that the /U/ vowel in “wood” is the same vowel as in “good,” the word sample shown for Oliver (Fig. 10).
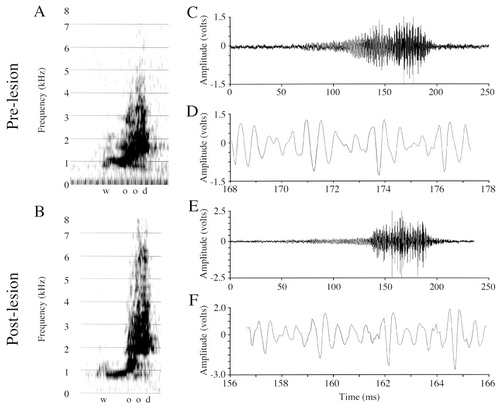
Spectrograms and amplitude waveforms of Peeper's prelesion and postlesion productions of “wood.” A: A prelesion production of “wood.” B: A postlesion production of “wood.” Note the very similar appearance between the prelesion and postlesion spectrograms and the lack of abnormal amplitude fluctuations (increases or decreases) in the postlesion spectrograms, especially in the vicinity of the /U/ (the same vowel sound as in Oliver's “good”). C: Amplitude waveform of the entire prelesion “wood” vocalization from A. D: A 9.5-msec excerpt from C. E: Amplitude waveform of the entire postlesion “wood” vocalization from B. F: A 9.5-msec excerpt from E. Note the normal appearance of the postlesion signal, and the lack of abnormal amplitude fluctuations (increases or decreases).
Amplitude.
Voltage measures of entire productions of “bear” did not reveal any significant differences prelesion and postlesion in maxV (t[21] = −1.02, P = 0.31), RMS (t[21] = −0.53, P = 0.60) or the maxV:RMS ratio (t[21] = −1.472, P = 0.15). Voltage measures of entire productions of “wood” did not reveal any significant differences prelesion and postlesion in maxV (t[15] = 1.640, P = 0.12) or the maxV:RMS ratio (t[15] = −1.55, P = 0.14), but RMS was significantly smaller postlesion (t[15] = 2.53, P < 0.03). Visual inspection of Peeper's “bear” and “wood” spectrograms and amplitude waveforms, however, did not reveal any abnormal amplitude fluctuations, either increases or failures, in any postlesion word samples.
Contact call analyses.
I did not detect any changes in Peeper's postlesion contact calls (Fig. 14A,B) auditorally or visually (by means of spectrograms), and visual inspection of the amplitude waveforms (Fig. 14C–F) did not reveal any abnormalities in the amplitude envelopes of postlesion calls. Spectrogram and amplitude waveform cross-correlations (Table 3) also failed to detect any differences in the similarity of the [pre × post] group compared with the [pre × pre] group (spectrogram cross-correlation, t[306] = −0.11, P = 0.91; amplitude waveform cross-correlation, t[341] = −0.90, P = 0.37). Indeed, both Peeper's spectrogram and waveform correlation indices increased postlesion [post x post], indicating that for contact calls, variability decreased, and stereotypy increased, postlesion ([pre × pre] vs. [post × post], for spectrograms, t[362] = −2.65, P < 0.01; for waveforms, t[371] = −4.28, P < 0.0001). Voltage measures of entire contact call samples (Table 2) revealed that all three measures were significantly greater postlesion (maxV t[38] = −5.37, P < 0.0001; RMS t[38] = −4.30, P = 0.0001; and maxV:RMS ratio t[38] = −2.61, P < 0.02).
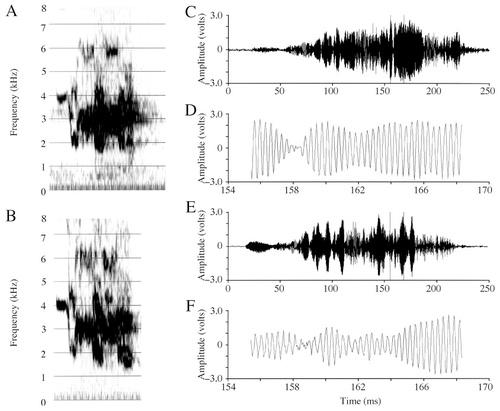
Spectrograms and amplitude waveforms of Peeper's prelesion and postlesion contact call productions. A: A prelesion contact call. B: A postlesion contact call. Note that the spectral shape is very similar between A and B, although not identical. C: Amplitude waveform of the prelesion contact call from A. D: A 14-msec excerpt from C. E: Amplitude waveform of the postlesion call from B. F: A 14-msec excerpt from E. Note that although waveforms are not identical, amplitude transitions in the postlesion signal are smooth.
In summary, neither Peeper's English vocalizations nor his contact calls exhibited auditorally or visually detectable abnormal amplitude fluctuations. For Peeper's English vocalizations, however, one of the amplitude measures (the RMS from “wood”) was significantly smaller postlesion than prelesion.
DISCUSSION
All birds that had any portion of NLc lesioned exhibited auditorally and visually detectable disruptions in their English vocalizations. In contrast, the control-lesioned bird did not exhibit disrupted English vocalizations. Below, I describe features of budgerigar vocalizations that are affected and unaffected by NLc lesions. I note which measures used to describe prelesion and postlesion vocalizations serve as useful indicators of the specific lesion effects. Results from this study are discussed in the context of a previously described model for budgerigar syringeal function, including the possible role of NLc. I discuss the implications of these findings for previous and future lesion studies in the budgerigar and other parrot species.
Summary of NLc lesion effects
NLc lesions affected production, but not memory, of learned English words and natural contact calls. By contrasting the terms “production” and “memory” I have attempted to emphasize the distinction between “motor” and other types of nonmotor memory, such as “declarative” memory, but do not intend to detract from the concept of motor memory as a valid memory type. Because I could find samples of all prelesion vocalizations in the birds' postlesion repertoires, I conclude that the birds' ability to recall words, i.e., their declarative memory for the words, remained intact. Instead, NLc lesions affected production of words. Another example might be helpful for clarifying this distinction: In my efforts to learn to speak French, I frequently realize that there are words that I have difficulty pronouncing, even though I know perfectly well which word it is that I intend to produce. For example, I can rarely produce the word “Monsieur” correctly. I know when to produce the word, I know what the word means, I even know how to spell the word. However, I cannot generate the correct motor commands to position my tongue and mouth to produce this word. Therefore, I conclude that my production of, but not memory for, the word “Monsieur” is poor. It is this simplified distinction between production and memory that I wish to extend to the vocalizations presented in this study.
The main effect of NLc lesions, then, was to affect production. Specifically, amplitude control in amplitude-modulated vocalizations was disrupted. Gross abnormalities were observed in the carrier signal amplitude of English vocalizations produced by all five birds that sustained NLc lesions. I observed three general categories of deficits: 1) abnormal control of the fine amplitude modulations normally seen in each amplitude-modulated period of a vocalization; 2) large, abnormal increases in amplitude of the carrier signals, modulating signals, or both; and 3) large, abnormal decreases in the amplitude of the carrier signals, modulating signals, or both (termed “failures”). NLc lesions also occasionally produced slight abnormalities in the frequencies of the carrier and modulating signals.
Analyses useful for identifying the effects of NLc lesions
English vocalizations.
I have identified empirical measures that are not only sensitive to the effects of NLc lesions, but that also describe, specifically, the fundamental differences between prelesion and postlesion vocalizations. I documented two measures (maxV and RMS), and their ratio (maxV:RMS) that describe how the amplitude of prelesion and postlesion English vocalizations differs quantitatively. Thus, the main effect of impaired fine amplitude control was to alter the relationship between the maxV and RMS, with greater ratios occurring postlesion (see Forest's “okay;” Table 2). Disruptions manifest as large amplitude increases resulted in a larger maxV and a larger RMS (see Mosaic's “paper,” the /e/ partial sample; Table 2). Disruptions that caused a decrease in the amplitude, or a failure, resulted in a smaller maxV and a smaller RMS (see Puck's “truck,” the /u/ partial sample; Table 2).
In contrast to the large, abnormal amplitude fluctuations observed in all five NLc-lesioned birds, Peeper, the control-lesioned bird, had no abnormal amplitude fluctuations in any of the hundreds of English vocalizations analyzed auditorally and visually. However, prelesion and postlesion amplitude measures comparing two of his words, “wood” and “bear” identified one amplitude measure (of six) that differed significantly postlesion (the postlesion RMS decreased for the /U/ sound in “wood;” Table 2). Considering the placement of a portion of Peeper's lesions in NF/NFl, lack of other abnormalities in his postlesion vocalizations is of particular interest. NFl has been implicated in the budgerigar vocal control pathway: it receives auditory projections from Bas, and it purportedly projects to NLc by means of NLs (Striedter, 1994). Thus, lesions in NFl might be expected to produce deficits in budgerigar vocalizations. A previous study by Hall et al. (1994) failed to identify effects of NF and NFl lesions on budgerigar contact calls, and this study further supports those findings.
For budgerigar vowel productions, then, NLc lesions, or lesions in the NLc-dependent pathway, are indicated by the combined presence of auditorally and/or visually detectable abnormal amplitude fluctuations and abnormal measures in one or more amplitude parameters (Table 3). These specific parameters, however, did not indicate disruptions produced by NLc lesions in budgerigar contact calls.
Contact calls.
The appropriate method for analyzing NLc lesion effects on contact calls was not initially obvious; therefore, prelesion and postlesion calls were compared in a manner similar to English vocalizations. Auditory assessment of the effects of NLc lesions on calls was inaccurate and usually insufficient: Postlesion changes were detected only for Forest's calls. Visual inspection of the amplitude waveforms identified changes in the postlesion contact calls of three NLc-lesioned birds, but were inconclusive for the other two (Table 3). Neither auditory nor visual (in the waveform) abnormalities were detected in the calls of the control-lesioned bird, Peeper.
Amplitude measures of contact calls (maxV, RMS, maxV:RMS) also yielded ambiguous results. Except for Grayson, all birds (including the control-lesioned bird, Peeper), had significant differences between prelesion and postlesion calls in two or more amplitude measures. Of interest, however, is the pattern of change in amplitude measures for the different birds. For Forest, whose contact calls were most severely affected, amplitude measures fluctuated in a manner similar to those of his postlesion English vocalizations. Thus, postlesion, his maxV was larger (although not significantly, likely due to the large variance), his RMS was significantly smaller, and his maxV:RMS ratio was significantly larger (Table 2). No other birds exhibited this same larger/smaller/larger pattern in the trio of amplitude measures. Observed in one bird, this pattern is not conclusive, but further studies examining the calls of birds with large NLc lesions may be able to determine whether this pattern can be used to reliably qualify or quantify the effects of NLc lesions on contact calls.
Contact call cross-correlation comparisons, in contrast, were highly predictive of which birds had received NLc lesions (Table 3). All five NLc-lesioned birds had significantly smaller similarity indices for prelesion and postlesion vocalization spectrogram cross-correlations compared with prelesion cross-correlated vocalizations. Four of five NLc-lesioned birds' had significantly smaller similarity indices when prelesion and postlesion vocalization waveforms were cross-correlated (only Mosaic's indices were not significantly different). In contrast, for the control bird, Peeper, postlesion spectrogram and waveform cross-correlations did not differ from prelesion cross-correlations. For contact calls, then, spectrogram and waveform cross-correlation differences, and perhaps the presence of visually detectable, abnormal amplitude fluctuations in the waveform, are indicative lesions in NLc or the NLc-dependent pathway.
Budgerigar word/vowel productions are thus a more sensitive assay than budgerigar calls for the effects of NLc lesions. Abnormalities were auditorally detected in the vowels of every budgerigar that had any portion of NLc lesioned. By using strictly auditory cues, postlesion differences were, in contrast, detected only in the calls of Forest. Although differences in prelesion and postlesion contact calls, presumably caused by NLc lesions, were detected by waveform and spectrogram cross-correlations, such analyses do not provide qualitative information about differences in prelesion and postlesion vocalizations. Visual analyses of amplitude waveforms of English vowels from birds with only minor NLc lesions revealed drastic abnormalities in carrier frequency amplitude, thus allowing both quantification and qualification of lesion effects.
Effects of NLc lesions on frequency
Because Forest's contact calls were audibly disrupted (in contrast to the calls of the other NLc-lesioned birds), I analyzed the frequency of the dominant (carrier) signal in his calls. No differences between the prelesion and postlesion dominant frequencies of his calls were identified. Because budgerigar contact calls have been regarded as frequency-modulated vocalizations, and little attention paid to their amplitude-modulated nature (Banta, 1998; Banta Lavenex, 1999), most researchers have tried to assess the effects of lesions on the dominant frequency of the contact call (Hall et al., 1994; Heaton, 1997). However, the present experiments show that damage to NLc, a large and highly interconnected vocal control nucleus (Fig. 1), does not disrupt the frequency of the dominant or carrier signal in contact calls. NLc lesions did, however, affect the amplitude at which the dominant or carrier signal was produced.
I analyzed the frequencies of the carrier and modulating signals in three vowel sounds in two different birds. Because Forest's lesions were the most extensive and produced the most drastic effects, I analyzed his /o/ and /e/ from “okay.” The carrier signal generated by Forest during these vowel productions was not decreased, and in some cases was even significantly faster postlesion. Because the large amplitude increases seen in Mosaic's postlesion “paper” productions simulated, to my ear, an increase in frequency, I also analyzed his /e/ from the “pa” syllable of “paper.” No significant differences in the carrier signal of the “pa” syllable were detected. The modulating signal generated by Forest during vowel productions was occasionally significantly slower or more difficult to identify postlesion (e.g., for the /e/ from “kay;” see Fig. 4E), but in most cases was present and maintained its periodic nature. For Mosaic's “pa” syllable from “paper,” no significant differences were observed between the frequency of the prelesion and postlesion modulating signal.
In addition to the increased carrier signal and decreased modulating signal frequencies sometimes observed in Forest's postlesion English vocalizations, complete failures of the carrier signal, modulating signal, or both, also occurred, especially during amplitude failures (e.g., in Puck's “truck”). Before drawing conclusions regarding the effects of NLc lesions on frequency, however, it is helpful to consider the relative severity of these disruptions (i.e., the increased and decreased frequencies), as well as the ultimate cause for both disruptions and failures (see below). Thus, taking into account that NLc lesions did not have any effects on the dominant frequency produced during contact calls and that the effects on the dominant frequency in English vocalizations were minor compared with the effects on amplitude, overall, the effects of NLc lesions on carrier signal frequency do not appear to be substantial. Furthermore, these minor postlesion frequency changes, as well as the more severe carrier and modulating signal failures, may actually be indirect effects of disrupted amplitude control. This possibility is discussed in further detail below.
Specificity of NLc lesions
Two additional points regarding the specificity of NLc lesion effects must be discussed: 1) Budgerigars' English vocalizations were tracked and analyzed (auditorally and visually on the Sona-graph) for months, and sometimes years, before the lesions. Amplitude fluctuations such as those presented here were never observed before the lesions. 2) A positive correlation exists between the amount of NLc lesioned and the severity of the observed vocal deficits. Forest's vocalizations were the most disrupted, and he sustained the largest NLc lesions (Table 1; Fig. 2). Mosaic, Puck, and Grayson had intermittent disruptions in their postlesion vocalizations, and they sustained small to intermediate lesions. Oliver's sustained the smallest NLc lesion of all birds, and his vocal deficits were almost undetectable, affecting only a very small number of his postlesion words. Grayson's NLc lesions, the second largest, affected much of the ventral border of NLc, rather than the center as for Mosaic and Puck, potentially accounting for why Grayson's vocal disruptions were less severe than might be predicted based solely on lesion size. Although there are, as of yet, no reports of differential functional topography of the NLc nucleus, it is nonetheless possible that such a functional topography exists. Indeed, the cells found along the ventral NLc border are more basophilic, compactly organized, and heterogeneous than other NLc neurons (Durand et al., 1997). Furthermore, by virtue of the very nature of a nuclear organization, it may be possible that neurons along the perimeter of a nucleus are less highly interconnected than those in the center, thus, potentially making perimeter lesions less effective than centrally located lesions. Finally, the control-lesioned bird, Peeper, exhibited no abnormal amplitude fluctuations in any of the postlesion vocalizations analyzed. Thus, both prelesion vocal behavior and a correlation between lesion size and the severity of effects provide further evidence for the specificity of NLc lesion effects.
Functional role of NLc
The present experiments suggest that budgerigar NLc, or the NLc-dependent pathway, is involved in the production of, but not memory for, learned vocalizations. NLc lesions did not cause the loss of elements (i.e., English words or contact calls) from the repertoire. Instead, NLc lesions affected acoustic features of budgerigar vocalizations, in particular the relative amplitude of the dominant or carrier signal, specifically associated with motor production. Furthermore, the intermittency of vocal disruptions in birds with small to intermediate sized lesions (Mosaic, Puck, Grayson, and Oliver) is also indicative of a motor disruption. It is known that relatively identical contractions of a single muscle group (i.e., identical in the amount of force produced and the effect on the target structure) may be accomplished by the activation of disparate groups of motor neurons (Tax et al., 1990). The lesion data presented here are consistent with this phenomenon. Thus, for example, approximately 55% of Mosaic's “paper” attempts were disrupted. Most likely, neurons affected by ibotenic acid were recruited to send signals to the syringeal muscles (by means of AAc and nXIIts), at some point during these abnormal productions. For the other 45% of Mosaic's “paper” productions, a relatively normal population of neurons was recruited, resulting in a normal “paper” production. Finally, several lines of evidence support the conclusion that NLc does not have a vocalization- or syllable-specific topography, and that effects of NLc lesions are not syllable specific: 1) Types of deficits were consistent across five different birds with five different NLc lesions, many different word types, and two different types of vocalizations (words and contact calls); 2) small NLc lesions produced only intermittent effects; and 3) large, incomplete lesions (i.e., in Forest, whose lesion occupied 49% and 34% of the right and left NLc, respectively), affected every vocalization similarly, and no vocalizations were unaffected.
Thus, to summarize, NLc lesions disrupt the amplitude at which budgerigar vocalizations are produced. However, amplitude is not simply increased, decreased, or prevented from varying throughout a vocalization. Rather, normal modulation of amplitude throughout the vocalization is disrupted. If NLc lesions disrupt normal modulation of amplitude, then the NLc-dependent pathway, and perhaps even NLc itself, must exert control over this feature in normal vocalizations. The amplitude of a vocalization is determined by the amount of air (i.e., the pressure of air) that passes between a set of vibrating membranes. At least two ways exist to vary the relative air pressure across a set of syringeal membranes: 1) vary the amount of air available to flow across the membranes; or 2) vary the syringeal aperture (the distance between the syringeal membranes) while maintaining the amount of air available to flow across the membranes. For the types and relatively rapid rates of amplitude modulation seen in budgerigar vocalizations, varying the amount of air available to flow across the syringeal membranes by means of direct activity of expiratory muscles does not seem to be a viable explanation. How, then, might syringeal mechanisms produce the amplitude modulations observed in the vocalizations of normal and NLc-lesioned birds? Envisioning a model for budgerigar syringeal function may elucidate these mechanisms.
Working model for budgerigar syringeal function
Based on acoustic analyses of words and contact calls, results from the present experiments, and previously published data (Heaton et al., 1995; Brittan-Powell et al., 1997; Heaton, 1997), I proposed a working model for budgerigar syringeal function (Banta, 1998; Banta Lavenex, 1999). Possibly the dominant or carrier frequency of budgerigar vocalizations is produced by a flow-induced, self-sustaining oscillation of the lateral tympaniform membranes (LTMs; achieved by Bernoulli action-like forces of air on the LTMs). The carrier frequency amplitude may then be modulated by either adducting or abducting the LTMs (i.e., moving them, respectively, into or out of the tracheal lumen, and, thus, into and out of the air flow). As the vibrating LTMs move in this manner (thus, increasing and decreasing the syringeal aperture), the amplitude of the sound produced would vary accordingly. Production of these two independent frequencies would thus make possible the production of vocalizations containing nonlinear amplitude modulation.
If such a model does explain the production of nonlinear amplitude modulation in the budgerigar syrinx, the effects of NLc lesions on budgerigar vocalizations support the hypothesis that amplitude modulation in the syrinx is centrally controlled. In all budgerigars, NLc lesions affected the amplitude at which the dominant or carrier signal was produced (resulting in amplitude increases or amplitude decreases). In terms of the proposed model, these effects would translate into an improper position of the LTMs in the lumen of the trachea caused by abnormal amplitude or movement of the vibrating LTMs (i.e., an abnormal modulating signal). Disrupted or impaired motion of the vibrating LTMs would also account for the slower modulating frequencies sometimes seen in Forest's vocalizations, as well as failures of the carrier signals, modulating signals, or both (e.g., if the membranes fail to enter the airstream, neither the carrier nor modulating signal would be produced). Note that although production of the carrier signal is dependent upon whether or not the membranes enter the airstream and, thus, is controlled by an NLc-dependent pathway, the frequency at which the carrier signal is produced does not seem to be regulated by means of an NLc-dependent pathway.
This model, although logical, does not define the structures or mechanisms responsible for moving the vibrating LTMs into and out of the tracheal lumen to create the modulating signal. Direct syringeal muscle activity, although potentially responsible, probably cannot explain all amplitude modulations present in budgerigar vocalizations. Amplitude modulation rates ranging from 100–742 Hz are common (Banta, 1998; Banta Lavenex, 1999), the latter being far greater than the rate at which even the fastest skeletal muscle can contract. Fee et al. (1998) recently proposed that nonlinear syringeal dynamics in the zebra finch (Taeniopygia guttata) are responsible for some nonlinear characteristics observed in its song, such as period doubling, mode-locking, and sudden transitions from periodic to aperiodic or chaotic signals. Similarly, nonlinear oscillations of the syringeal membranes may produce amplitude modulation in budgerigar vocalizations. If so, the lesion data presented here suggest that these nonlinear dynamics must be under some form of central control and that lesion of NLc can disrupt specifically the magnitude of the nonlinear syringeal oscillations.
Finally, this study provides evidence for the ubiquitous presence of nonlinear amplitude modulation in natural budgerigar contact calls. Previously, acoustic analyses were able to identify conclusively the presence of nonlinear amplitude modulation in only a few specific regions of contact calls where a complex array of frequency components exists (i.e., where periodic amplitude modulation is present; Banta Lavenex, 1999). Throughout the remainder of the call, the spectrum is simply broadband. Inspection of amplitude envelopes and waveforms from entire contact calls reveals numerous, drastic amplitude changes throughout; but what cannot be ascertained from acoustic analyses is whether these frequent changes in amplitude arise from nonlinear, aperiodic amplitude modulation (in contrast to periodic amplitude modulation; Banta Lavenex, 1999). These experiments show that amplitude throughout the entire contact call is similarly affected after NLc lesions (see Forest's postlesion call; Fig. 5). Thus, convergent evidence suggests that a majority of the acoustic properties of budgerigar contact calls arise from nonlinear amplitude modulation: First, the acoustic spectra of budgerigar productions of human vowels and some regions of contact calls arise from nonlinear amplitude modulation (Banta Lavenex, 1999). Second, the amplitude at which the carrier signal is modulated in budgerigar vowels is affected by NLc lesions (Banta, 1998; this study). Thus, broadband regions of contact calls that are similarly affected are likely to be produced by the same physical mechanism as budgerigar vowels, i.e., by a process of nonlinear amplitude modulation (be it periodic or aperiodic modulation). Clearly, further physiological experiments are needed to test these hypotheses and elucidate the role of the syrinx and central vocal control nuclei in the production of these amplitude-modulated vocalizations.
Further implications of amplitude modulation for investigations of the neural substrates underlying budgerigar vocal production
Recognizing the presence of amplitude modulation in budgerigar vocalizations may aid in interpreting effects of lesions in other vocal control nuclei. Hall et al. (1994) lesioned budgerigars unilaterally and bilaterally in and around Field “L” and Bas. The authors found no effects after Field “L” lesions, but Bas lesions were noted to cause deterioration, loss of individual distinctiveness, and loss of all frequency modulation (determined by visual inspection of Fourier spectra) of contact calls. What is not known, however, is how their lesions affected the gross temporal envelope, amplitude waveform, or amplitude modulation present in these vocalizations.
Heaton (1997) similarly relied on inspection of spectrograms and comparisons of prelesion and postlesion dominant frequencies (the frequency at which the greatest amount of energy in the spectrum occurs) to assess lesions effects. He suggests, from analyses based on these techniques, that bilateral lesion of AAc results in a decrease of the dominant frequency of budgerigar contact call productions, and thus, that AAc lesions are directly responsible for disrupting tension on the syringeal membranes (the LTMs), thus, decreasing the frequency at which they can vibrate during vocalization. However, contact calls of bilaterally AAc-lesioned birds, unlike those of unlesioned birds, were essentially harmonic vocalizations, consisting of a fundamental frequency and its associated series of harmonics. It is possible, if not probable, that calls of AAc-lesioned birds are similar to those harmonic calls produced by bilaterally denervated birds from the study by Heaton et al. (1995). Brittan-Powell et al. (1997) showed that the dominant frequency of the harmonic calls produced by bilaterally denervated birds shifted when produced in helium, whereas calls of normally innervated birds did not. The data suggest that these harmonic calls, whether the result of AAc lesion or bilateral syringeal denervation, are, in general, produced in a manner fundamentally different from those of normal birds. Heaton (1997) did not address the implications of the results of the helium experiments when presenting the effects of AAc lesions on contact calls. Furthermore, he relied exclusively on visual analyses of spectrograms and measures of the dominant frequency for his acoustic comparisons of prelesion and postlesion vocalizations. What is not known is how the presence and pattern of amplitude modulation were affected in AAc-lesioned birds. Consideration of the acoustic implications of amplitude modulation should facilitate more thorough and accurate investigations of the effects of lesions in budgerigar vocal control nuclei.
CONCLUSIONS
These experiments show that lesions in the budgerigar vocal control nucleus, NLc, or the NLc-dependent pathway, affect production, but not memory, of learned English and natural vocalizations. NLc lesions disrupted, specifically, the amplitude at which the carrier frequency of amplitude-modulated vocalizations was produced. Importantly, NLc lesions did not affect the frequency at which the dominant signal was produced. The effects of NLc lesions on amplitude implicate nonlinear amplitude modulation as a key feature of contact calls. Future investigations of the function of the budgerigar vocal control system will benefit from taking into account the presence and acoustic implications of nonlinear, periodic and aperiodic amplitude modulation in budgerigar vocalizations.
These experiments raise questions regarding the functional role of the NLc-dependent pathway in other parrot species. For the budgerigar, amplitude regulation seems to be a critical feature of much of its vocal behavior, from amplitude-modulated calls to the “loud” and “soft” warble produced in various behavioral contexts (Brockway, 1964; Farabaugh et al., 1992). Is amplitude regulation important for all parrot species, and, if so, does the NLc-dependent pathway also play a role in amplitude control in these other species? Interestingly, the Grey parrot also produces a range of sounds of greatly differing amplitudes, as well as some amplitude-modulated vocalizations (Banta, personal observation). Grey parrots also have a central vocal control system that is similarly arranged to that of the budgerigar, with laterally adjacent NLc and AAc nuclei (Banta, personal observation). Future research must probe the acoustic, structural, and neuroanatomic bases of vocal behavior in other parrot species to know how much we can generalize about parrot vocal behavior from studies of the budgerigar.
Acknowledgements
I thank I.M. Pepperberg, R.R. Capranica, and P. Lavenex for the invaluable help and guidance they have given me throughout this study, and P. Lavenex and I.M. Pepperberg for comments on previous versions of this manuscript. I thank S. Bottjer, G. Striedter, and B. McNaughton for invaluable help and advice regarding the lesions presented here. I thank numerous excellent undergraduate students at the University of Arizona for training budgerigars, and S. Rubin and L. Freeman for superb care of the breeding flock of budgerigars. I thank B. Brittan-Powell, F. Goller, and R. Suthers for helpful discussions. I thank T. Glattke, S. Hopp, and P. Marler for helpful discussions and access to equipment necessary to complete this study, and D. Amaral and B. McNaughton for access to equipment. I.M. Pepperberg was supported by the Whitehall Foundation and the National Science Foundation.




