Support for linkage of autism and specific language impairment to 7q3 from two chromosome rearrangements involving band 7q31
Abstract
Childhood autism is characterised by impairments in communication and reciprocal social interaction together with restricted/stereotyped interests, which are evident before 3 years of age. Specific developmental disorders of speech and language (SDDSL) are characterised by impairment in the development of expressive and/or receptive language skills which is not associated with intellectual, sensory, physical, or neurological impairment. Family and twin studies indicate a substantial genetic component in the aetiology of both disorders. They also reveal increased rates of SDDSL in relatives of autistic individuals, suggesting that this phenotype can represent one manifestation of the genetic liability for autism. Modelling of the recurrence risk for autism and milder phenotypes, such as SDDSL, suggest that three or four epistatic loci may be aetiologically involved. A recently published linkage study of an exceptional family with an apparently dominantly inherited SDDSL implicated chromosome band 7q31 as the site of the putative susceptibility locus (SPCH1). This region of chromosome 7 also shows strong linkage in multiplex families with autism. We present two individuals (one has autism, the other SDDSL) with different, apparently balanced chromosome rearrangements involving a breakpoint at 7q31.3. Fluorescence in situ hybridisation was used to localise the breakpoints to an ∼1 cM interval between CFTR and D7S643. Our findings may be of interest and relevance to the genetic aetiology of autism, and helpful in the search for susceptibility loci for SDDSL and autism. Am. J. Med. Genet. (Neuropsychiatr. Genet.) 96:228–234, 2000. © 2000 Wiley-Liss, Inc.
INTRODUCTION
There has been a long-standing debate over the phenotypic overlap between individuals diagnosed with autism, other pervasive developmental disorders, and specific developmental disorders of speech and language (SDDSL) (the term SDDSL encompasses disorders commonly referred to as specific language impairment or SLI) [Bishop, 1994]. Family and twin studies of both disorders indicate a genetic component to their aetiology [Bolton et al., 1994; Bailey et al., 1995; Tallal, 1989; Bishop et al., 1995].
The most recent British twin study of autism gave an estimate of the heritability of autism of 91–93%. Where there was discordance in phenotype between co-twins, specific cognitive abnormalities that included specific language impairments were found in 50% of nonautistic monozygotic co-twins, and in 10% of nonautistic dizygotic co-twins [Bailey et al., 1995]. These results, together with findings from family studies, suggest that some SDDSL may represent a variable manifestation of the genetic liability to autism [Bailey, 1998].
A large-scale twin study of SDDSL [Bishop et al., 1995] showed substantial heritability for two subtypes of SDDSL: expressive language impairment and expressive language impairment with an articulation disorder. The results of this study also suggest that SDDSL are not genetically distinct from less specific disorders involving language impairment in the context of low-average or borderline nonverbal ability. Some degree of more general learning difficulties may therefore exist in a proportion of SDDSL children [Bishop, 1994]. Approximately 80% of autistic individuals have intellectual impairment [Fombonne, 1999].
The underlying neuropathological basis of autism and SDDSL and the relationship between them is currently unknown. Most multiplex families with autism do not show a simple pattern of inheritance [Pickles et al., 1995]. Inheritance patterns observed in multiplex families with SDDSL do not usually fit a Mendelian pattern of inheritance [Bishop et al., 1995]. Nevertheless, one large family (the KE family) has been reported with an SDDSL that persists in adulthood and is inherited in an autosomal dominant manner [Hurst, 1990]. Affected individuals have verbal and orofacial dyspraxia and language impairment, with expressive language skills more severely impaired than receptive language. Broader cognitive impairments, resulting in low-average and borderline full-scale IQ, are also present [Vargha-Khadem et al., 1995]. Both functional and structural abnormalities have been found in several areas of the brain of affected individuals [Vargha-Khadem et al., 1998].
Linkage study of the KE family [Fisher et al., 1998] found strong linkage to chromosome band 7q31. Multipoint mapping indicated that the 3 lod unit confidence interval for the location of the putative gene responsible (SPCH1) was a 5.6 cM region in 7q31 between D7S2459 and D7S643. The 3.8 cM interval between D7S2425 and the gene CFTR (cystic fibrosis transmembrane conductance regulator) produced a peak multipoint lod score (MLS) of 6.62. The authors postulated a single locus with full penetrance was responsible for the SDDSL, although a microdeletion involving several contiguous genes could not be discounted. Preliminary data from association studies of SDDSL and markers in 7q31 have provided some tentative support for an association between CFTR and SDDSL in a wider population of children with SDDSL [Tomblin et al., 1998].
The first genome-wide linkage study of autism [IMGSAC, 1998] produced the most positive linkage results implicating chromosome 7 in the search for susceptibility genes for autism. A peak multipoint lod score (MLS) of 2.53 was found near markers D7S530 and D7S684 at 7q31-35, although a large (40 cM) region between D7S527 and D7S483 (7q22-7q35) showed significant linkage. It is noteworthy that the IMGSAC used a restricted definition of the autistic phenotype, such that language impairment was more severe than that required for diagnosis by the fourth revision of the Diagnostic and Statistical Manual, DSM IV [APA, 1994] or the Autism Diagnostic Interview-Revised (ADI-R) [Lord et al., 1994].
Other linkage studies of autism have produced positive results for 7q3. One study found a peak MLS of 0.83 for D7S486, which maps at 7q31 [Philippe et al., 1999], another has reported a peak MLS of 1.77 at D7S2527 [Ashley-Koch et al., 1999]. A peak MLS of 0.93 at D7S1804 was reported by the study of Risch et al. [1999].
All linkage studies of autism have also yielded positive results for other chromosomal regions, indicating other candidate regions for susceptibility loci. Furthermore, the association of some cases of autism with certain medical conditions such as Fragile X syndrome and tuberous sclerosis indicates some aetiological heterogeneity for the disorder. [Fombonne, 1999].
CLINICAL AND CYTOGENETIC FINDINGS
Case 1
Case 1 was born at term to healthy, unrelated parents. His early developmental milestones were delayed; he walked at 17 months by parental report. He underwent initial assessment at 20 months of age, where he scored an age equivalent of 11–12 months on the Griffiths Developmental Scales [Griffiths, 1986] and all scores of words and gestures on the McArthur [Fenson et al., 1993] were at a <8 month level. He met the ICD-10 [WHO, 1993] diagnostic criteria for autism on clinical examination.
On recent assessment at 31 months of age he scored a nonverbal IQ age equivalent of 22 months. He has no speech and meets all criteria for autism on the ADI-R [Lord et al., 1994] and the Autism Diagnostic Observation Schedule (ADOS) [Lord et al., 1989]. Thus, his clinical profile indicates significant delay in all areas of development, with disproportionate impairment of communication skills and behaviours that meet the strict diagnostic criteria for autism. Case 1 has a maternal uncle with Asperger syndrome (a syndrome qualitatively similar to autism, with impairment in social interaction and relationships in the apparent absence of early language difficulties) [Volkmar et al., 1998] and a cousin with SDDSL (Fig. 1). An apparently balanced de novo pericentric inversion of chromosome 7 was found on chromosome analysis (Fig. 2a).
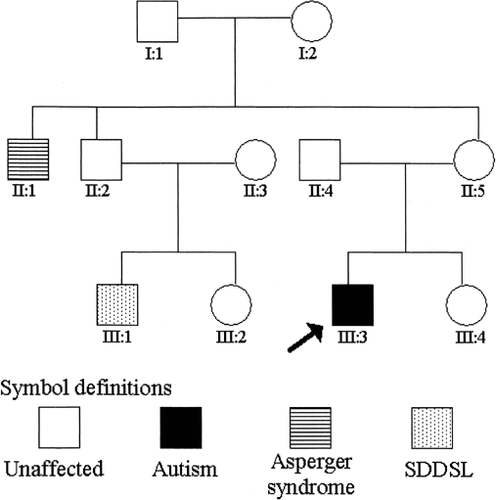
Pedigree for Case 1.
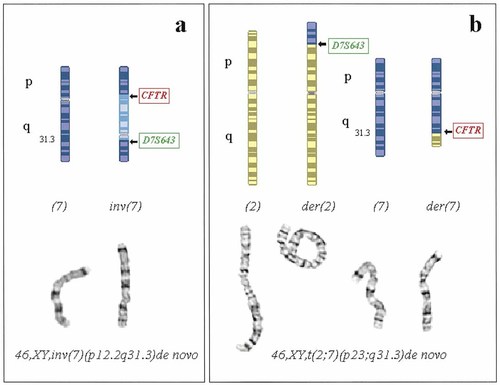
Computer-generated colour ideograms [Scriven, 1998] and images from G-banded metaphase preparations of the chromosome rearrangements seen in (a) Case 1, (b) Case 2. A full ISCN [ISCN, 1995] description of each karyotype is given. The position of probes containing CFTR and D7S643 is shown on the ideograms for the rearranged chromosomes.
Case 2
Case 2 was born after an uneventful pregnancy at 38 weeks by normal delivery to healthy, unrelated parents. He had no neonatal complications. His motor development was normal; he sat at 7 months and walked at 11 months of age. He had only developed a few single words by the age of 2 years and was 3½ before he put words together. A hearing assessment by an audiologist was normal at age 4 years 5 months.
Case 2 was an active toddler with oppositional behaviour. He had difficulties with peer relationships at nursery school and required full-time educational support in mainstream primary school. He was given a diagnosis of oppositional defiant disorder at age 5 years. He was also described as shy and emotionally immature.
Assessment with the Griffiths Developmental Scales [Griffiths, 1986] at age 4 years 10 months indicated that he was functioning at the low end of the average range. Assessment of language skills at 5 years of age using the Reynell Developmental Language Scale [Reynell, 1985] showed age-appropriate comprehension but difficulties in expressive language and speech sound production. Assessment with the Weschler Preschool Primary Scale of Intelligence (WPPSI) [Weschler, 1990] at age 5 years 5 months revealed a 10-point verbal discrepancy with lower language scores. This did not reach statistical significance. His overall IQ was 74. He scored an age equivalent of <5 years on both the receptive and expressive scales of the Clinical Evaluation of Language Fundamentals-Revised (CELF-R) [Semel et al., 1987] at age 6 years 3 months.
At 6 years 8 months Case 2 continued to have difficulty following verbal instructions in school. He was described as having a good vocabulary but had difficulty expressing his ideas. He continues to show word finding difficulties, word sequencing difficulties, and poor articulation. An ADI-R [Lord et al., 1994] and the ADOS [Lord et al., 1989] excluded a diagnosis of pervasive developmental disorder. On examination, Case 2 was right-handed and right-footed. EEG examinations were normal. An MRI scan, carried out when Case 2 was 6 years 11 months, revealed an anomaly, likely a small dysembryoplastic neuroepithelial tumour (DNET) in the temporal horn of his right hemisphere.
Reports of Case 2's development thus describe disordered language development, in the context of normal physical/motor development, and an overall IQ in the low-average range. He also has significant attention and behavioural problems. His mother has a history of bipolar affective disorder. A reported history of delayed language development elsewhere in the maternal side of the family could not be reliably confirmed. An apparently balanced de novo reciprocal translocation between chromosomes 2 and 7 was found on chromosome analysis (Fig. 2b).
MATERIALS AND METHODS
Cytogenetic Studies
Chromosome analysis was performed on GTG-banded metaphase preparations from cultures of PHA stimulated, thymidine synchronised, peripheral blood lymphocytes using standard cytogenetic techniques.
Molecular Cytogenetic Studies
The 7q31.3 breakpoint of our two chromosome rearrangements was mapped using fluorescence in situ hybridisation (FISH). Probes were produced from YAC clones containing the genetic markers showing strongest linkage in the KE family study.
YAC clones containing D7S643 (881_c_6) and D7S633 and D7S677 (751_e_9) were obtained from the CEPH Mega YAC library of the UK MRC HGMP Resource Centre. HSC7E127, (which contains D7S677 and the CFTR gene inclusive of exon 24) clone and probe were kindly supplied by Dr. S. Scherer and Dr. S. Tosi. FISH probes were prepared from the clones as previously described by Davies et al. [1995]. Nick translation was carried out using a commercial “Bio-Nick” kit (Life Technologies, Bethesda, MD). The protocol of Davies et al. [1995] was used for hybridization and detection of noncommercial probes. A commercial probe was obtained for D7S486 (Vysis) and applied in accordance with the manufacturer's instructions.
RESULTS
Successful FISH results were obtained for D7S486, 751_e_9 (D7S633 and D7S677), HSC7E127 (D7S677 and the CFTR gene inclusive of exon 24), and 881_c_6 (D7S643). All of these STS markers map at 145 cumulative cM from the top of chromosome 7 [Bouffard et al., 1997]. The latter two probes map the 7q31.3 breakpoint of both patients to ∼1 cM stretch of DNA between these two markers (Fig. 3). The breakpoints are in a position consistent with significance in relation to the linkage study data (Fig. 4). The linkage results from the KE family gave a peak multipoint lod score of 6.62 between D7S2459 (140 cM) and D7S643 (145 cM). Markers at 145 cM (D7S486 and CFTR) gave maximal pairwise lod scores of 6.22 and 5.46, respectively [Fisher et al., 1998]. The Phillippe et al. [1999] study of autism found a peak MLS of 0.83 at D7S486 at 145 cM. The study of autism reported by Ashley-Koch et al. [1999] found an MLS peak of 1.77 at D7S2527, ∼6 cM distal at 151 cM. The IMGSAC [1998] study of autism reported peak linkage results 12–24 cM distal to the breakpoints, near D7S530 and D7S684. The study of Risch et al. [1999] reported a peak MLS for D7S1804, 10.3 cM proximal to the IMGSAC peak.

FISH with probes constructed from YACs: HSC7E127 (CFTR) (a,c) and 881_c_6 (D7S643) (b,d). Images (a) and (b) show partial chromosome spreads from Case 1; the inverted chromosome 7 is indicated by a long arrow, the normal chromosome 7 by an arrowhead. Images (c) and (d) show partial chromosome spreads from Case 2; derivative chromosomes 2 and 7 are depicted by long arrows, their normal homologues by arrowheads. The identity of the chromosomes in all four images was confirmed by enhancement using PSI Macprobe Powergene software to produce banded chromosomes.
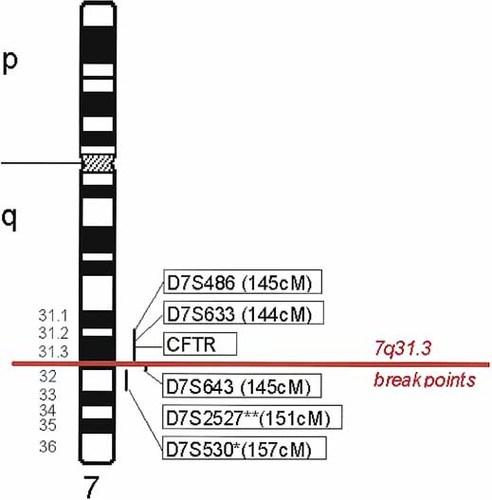
Ideogram of chromosome 7 showing the position of the 7q31.3 breakpoints relative to current mapping data for markers showing peak multipoint lod scores in published linkage studies of autism and SDDSL. The proximal-distal order of markers was obtained from the physical map of Bouffard et al. [1997, online version]; their genetic positions are shown in brackets. The cytogenetic position of clones containing markers used in this study and determined by our analysis, is also shown. *The cytogenetic position of D7S530 was obtained from published work [Korenberg et al., 1999]. **No cytogenetic mapping data were available for D7S2527.
DISCUSSION
The finding of two patients with related phenotypes both with a de novo chromosome breakpoint which maps within a ∼1 cM stretch of DNA in chromosome band 7q31.3 is very interesting. Apparently balanced chromosome rearrangements that disrupt gene function at their breakpoints have contributed significantly to the mapping of disease genes. Though a causal role for either of the 7q31.3 breakpoints cannot be concluded, the chance of a false-positive association is reduced in the presence of other supportive genetic data.
The concordance between the position of the 7q31.3 breakpoints reported here with the linkage study data published to date is striking: three studies to date map within 6 cM of the breakpoints [Fisher et al., 1998, Ashley-Koch et al., 1999; Phillippe et al., 1999]. The IMGSAC [1998] reported peak linkage was 12–24 cM distal to the breakpoints (though positive results were obtained for CFTR and D7S486). Precedents from the study of other conditions suggest a complex relationship between the site of disruption by chromosome breakpoints and the position of the affected gene; chromosome rearrangements have been mapped up to ∼1 Mb upstream from the gene they affect [Pfeifer et al., 1999]. Thus, the discrepancy between the breakpoints' position and position of peak linkage observed by the IMGSAC does not exclude the possibility that both findings reflect the presence of a single key susceptibility locus in 7q3. Alternatively, a locus distal to 7q31.3 may be responsible for the linkage results; the 7q31.3 breakpoint may then represent disruption of a second susceptibility locus, or a spurious finding.
Given the well-established clinical association between autism and SDDSL, the proximity of Case 1's chromosome breakpoint to both the putative locus responsible for the SDDSL of the KE family (SPCH1), and within ∼1 cM of Case 2's breakpoint is intriguing. Its position suggests that a common locus (which may be SPCH1) is implicated in the aetiology of both SDDSL and autism, although two or more closely linked or contiguous genes that contribute to the different phenotypes cannot be excluded.
A recent report of a family with a maternally inherited inversion of chromosome 7 implicated the more proximal chromosome band 7q31.2 in the aetiology of both autism and SDDSL. The two male siblings of this family have autism, while their sister has significant developmental delay and an expressive language disorder [Ashley-Koch et al., 1999]. Imprinting was postulated to contribute to the complex inheritance pattern observed in this family, although a coincidental role for this breakpoint cannot be discounted.
Collectively, our findings and results published to date implicate chromosome band 7q31 in the aetiology of autism and SDDSL and suggest the presence of one or more susceptibility loci in or close to this region of chromosome 7.
The clinical findings for Case 1 are straightforward. The pedigree is consistent with studies of inheritance patterns of autism and related phenotypes recurring in families, which suggest the action of several loci interacting in a multiplicative manner. Mathematical modeling of recurrence risks in multiplex families with autism suggests a multiple locus model of inheritance, with three loci producing the best fit with the data, but with up to 10 loci possible [Pickles et al., 1995]. Models postulating a large number of susceptibility loci have also been proposed [Risch et al., 1999].
The presence of other family members with related, milder phenotypes (SDDSL and Asperger syndrome) in the pedigree of Case 1 is consistent with disruption of a susceptibility locus at 7q31.3 in addition to the inheritance of one or more susceptibility genes segregating in the maternal side of the family (which could include mutation at the same locus in the other homologue of chromosome 7).
The pedigree also raises intriguing questions about the contribution of the susceptibility loci to various aspects of the autistic phenotype: does the Asperger syndrome of Case 1's maternal uncle suggest possible segregation of the social/behavioural and verbal communication aspects of the autistic phenotype?
Case 2's clinical picture is complicated by the recent diagnosis of a probable dysembryoplastic neuroepithelial tumour (DNET) in his right hemisphere. However, given that he shows left hemisphere dominance, his DNET is unlikely to be the primary cause of his language difficulties, although it may contribute to his overall phenotype.
Reports of increased rates of depression and social phobia in the relatives of autistic probands [Bailey, 1998] may suggest the presence of one or more susceptibility loci in Case 2's mother, although a recent study did not find this tendency in relatives of probands with “broad autistic phenotypes” [Piven and Palmer, 1999]. However, difficulties in phenotype definition, the changing presentation of SDDSL with age, and inadequate tools for the measurement of residual language difficulties in adults mean that a single major gene effect for at least some SDDSL cannot be excluded at present. It is possible, therefore, that Case 2's SDDSL may be a result solely of the 7q31.3 breakpoint acting in a dominant manner.
It is interesting that the phenotypes of both Case 2 and the KE family fall within the “AE subtype” (articulation plus expressive language disorder with or without receptive language disorder) described by Bishop et al. [1995] in their twin study of SDDSL, since the results of that study indicated that this SDDSL phenotype was one of two that showed strong evidence of heritability.
This report describes the localisation of two chromosome breakpoints occurring in 7q31.3 which map to an ∼1 cM stretch of DNA, implicated by linkage study in the aetiology of autism and SDDSL. The breakpoints are therefore of interest in the search for susceptibility genes for these disorders, particularly since they may implicate a common locus (and possibly SPCH1) in the aetiology of both autism and SDDSL.
Acknowledgements
We thank our two cases and their parents for their co-operation. We thank Ghazala Mirza and Angela Davies for help with construction of probes and Ioannis Ragoussis for useful discussions on the mapping analysis. Thanks also to Simon Fisher, Anthony Monaco, and Anthony Bailey for their helpful comments on the manuscript and Paul Scriven for help with its compilation.




