Bipolar disorder and variation at a common polymorphism (A1832G) within exon 8 of the Wolfram gene
Abstract
A number of linkage studies provide evidence consistent with the existence of a bipolar susceptibility gene on chromosome 4p16. The gene for Wolfram syndrome, a rare recessive neurodegenerative disorder, lies in this region and has recently been cloned. Psychiatric disturbances including psychosis, mood disorder, and suicide have been reported at increased frequency in Wolfram patients and in heterozygous carriers of a Wolfram mutation. In the current investigation we have undertaken a case-control association study using a single nucleotide polymorphism (causing an amino acid change) in exon 8 of the Wolfram gene in a UK Caucasian sample of 312 Diagnostic and Statistical Manual of Mental Disorders (fourth edition; DSM IV) bipolar I probands and 301 comparison individuals. We found no evidence that variation at this polymorphism influences susceptibility to bipolar disorder. It remains possible that variation at other sites within or near the Wolfram gene plays important roles in determining susceptibility to affective illness. Am. J. Med. Genet. (Neuropsychiatr. Genet.) 96:154–157, 2000. © 2000 Wiley-Liss, Inc.
INTRODUCTION
Family, twin, and adoption studies indicate that genetic factors influence susceptibility to bipolar disorder [Craddock and Jones, 1999], but the mode of inheritance is complex. Although a few families may exist in which a single gene determines susceptibility to illness, the pattern of inheritance of the majority of bipolar disorder in the population is consistent with several interacting genes or with more complicated genetic mechanisms [Craddock et al., 1995].
Wolfram syndrome [Wolfram and Wagener, 1938] is a recessive neurodegenerative disorder, with a prevalence of 1 per 770,000 in the United Kingdom [Barrett et al., 1995]. The characteristic symptoms are juvenile onset diabetes mellitus and progressive bilateral optic atrophy. Patients may later develop diabetes insipidus and deafness, as well as a range of neurologic and psychiatric abnormalities, including dementia, psychosis, affective disorder, suicidal tendencies, and violent behavior. An excess of psychiatric hospitalizations and suicides has been reported in the blood relatives of patients suffering from Wolfram syndrome, an observation that led to the suggestion that heterozygous carriers of the gene for Wolfram syndrome are predisposed to psychiatric illness [Swift et al.,1991]. The same group reported that the proportion of psychiatrically hospitalized relatives who carried the Wolfram gene was greater than the number expected by chance [Swift et al., 1998]. The authors estimated that carriers were 26 times more likely to require psychiatric hospitalization than noncarriers, and suggested that heterozygote carriers of the gene might constitute as much as 25% of all persons hospitalized with similar psychiatric disorders.
The gene for Wolfram syndrome, WFS1/ Wolframin, recently has been identified on chromosome 4p16 [Inoue et al., 1998; Strom et al., 1998]. A large number of different mutations in WFS1 can cause the syndrome [Hardy et al., 1999; Inoue et al., 1998; Strom et al., 1998]. The Wolfram gene cDNA is 3,688 base pairs in length and is composed of eight exons. The sequence predicts a polypeptide of 890 amino acids, with hydrophilic N- and C-terminals and a central hydrophobic core of approximately 350 residues. The deduced sequence suggests the presence of about nine transmembrane segments. It is expressed in a variety of tissues, including heart, brain, placenta, lung, and pancreas [Strom et al., 1998]. Its exact function is unknown at present but it appears to be involved in maintenance of normal islet β-cell function [Inoue et al., 1998].
Interestingly, linkage studies of bipolar disorder implicate this region as harboring a putative bipolar susceptibility gene. Blackwood et al. [1996] reported a large Scottish pedigree that provided a maximum lod score of 4.8 with markers across this 4p16 region. Asherson et al. [1998] have described a schizoaffective pedigree with bipolar features that shows linkage (lod score 1.94) with markers in the same region, and three other groups have presented supportive linkage evidence with markers in the region [Kennedy and Macciardi, 1998].
A parsimonious explanation for the above findings would be that variation in the Wolfram gene itself might influence susceptibility to bipolar disorder. There are two mechanisms (not mutually exclusive) by which variation at the Wolfram gene might have this effect. First, as hypothesised by Swift, heterozygous carriage of mutations that give rise to Wolfram syndrome in the homozygous form could predispose to psychiatric disorders. Second, a variant of the Wolfram gene might exist, which in either homozygous or heterozygous form is insufficient to cause Wolfram syndrome itself, but might predispose carriers to psychiatric illness. We have observed no compelling evidence in support of the former hypothesis in our sample of 29 U.K. Wolfram pedigrees [Barrett et al., 1995]. To investigate the latter hypothesis we have conducted a case-control association study for a common polymorphism in the Wolfram gene in a group of bipolar patients and comparison groups.
MATERIALS AND METHODS
A full mutational analysis has been performed on a large panel of 30 Wolfram's patients and four controls [Hardy et al., 1999]. The polymorphism investigated in this report occurs in exon 8 of the Wolfram gene [Inoue et al., 1998], within which the majority of 25 mutations also have been found, thus any amino acid change, such as that created by the polymorphism studied, could have an important functional consequence. The polymorphism selected was one of 14. It is caused by the substitution of A to G at position 1832, causing an amino acid change of Arg to His at amino acid residue 611. The change creates an Hha1 restriction site. This polymorphism was chosen for study because it causes an amino acid change and has approximately equifrequent alleles. The former property increases the likelihood that the polymorphism could itself influence disease susceptibility; the latter property maximizes the power of a sample of fixed size for detecting a disease-allele association. We have designed a rapid polymerase chain reaction (PCR) screening protocol to determine genotypes at this polymorphism in 314 patients with bipolar disorder and 301 control subjects.
Samples
U.K. Caucasian subjects with Diagnostic and Statistical Manual of Mental Disorders (fourth edition; DSM IV) [American Psychiatric Association, 1994] bipolar I disorder (n=312, mean age 47 ± 26 SD, 58% female), were recruited from outpatient clinics in Wales and the Midlands of England. Probands were all interviewed by a trained psychiatrist using either SADS-L (Schedule for Affective Disorders and Schizophrenia, Lifetime Version) [Endicott and Spitzer, 1978] modified to provide diagnostic information for DSM IV or by the Schedule for Clinical Assessment in Neuropsychiatry (SCAN) [Wing et al., 1990], and hospital records were obtained. Best estimate lifetime diagnoses were made on the basis of all available clinical data. Unrelated British Caucasian comparison individuals, who were not screened to exclude subjects with a history of psychiatric illness, were recruited from the Blood Transfusion Service in South Wales (Group 1: n=191, mean age 44 ± 10 SD, 58% female) or were patients attending a family medical practitioner in South Wales for nonpsychiatric reasons (Group 2: n=110, mean age 48 ± 18 SD, 65% female). Ethical approval was obtained for the study and all subjects provided written informed consent to participate in genetic studies.
Genotyping
High molecular weight DNA was extracted from whole blood according to routine procedures. A 477 bp fragment was amplified by PCR using forward primer WFSF: 5′ TTC CGC ATG GCA CAG CTG AGG 3′ and reverse primer WFSR: 5′ CCC GCA CAG CGC ACC ATA CTG 3′. Each reaction consisted of an initial denaturation at 94°C for 5 min followed by 35 cycles of 45-sec denaturation at 94°C, 45 sec at the annealing temperature of 65°C, and 45 sec primer extension at 72°C. This was followed by a final extension at 72°C for 5 min.
Each 12.5 μL reaction contained 50 ng genomic DNA, 5 pmol of each primer (Alta Bioscience, Birmingham, U.K.), 1.0 mM MgCl2, 200 μ M dNTPs, 0.5 units Taq polymerase (Bioline, London, U.K.), and 1× Taq buffer (as supplied by manufacturer).
The PCR product was digested for 18 hours with 4 units HhaI restriction endonuclease (New England Biolabs (U.K.) Ltd, Hitchin, Hertfordshire, U.K.). The fragments were separated on 2.5% agarose gel and visualized with ethidium bromide.
The 477 bp PCR product contained two nonpolymorphic restriction sites at positions 1675 and 2013. The former was cut in all samples, whereas cleavage at the latter produced a fragment too small to resolve. Cleavage at the polymorphic site at position 1832 was dependent on the presence of G rather than A at the polymorphism. It was possible to resolve two fragments, 338 and 130 bp, from the digestion of genotype AA. Three fragments, 181, 157, and 130 bp, were resolvable from the digestion of genotype GG, and four from heterozygotes AG: 338, 181, 157, and 130 bp. A visualized gel giving examples of each genotype is shown in Figure 1.
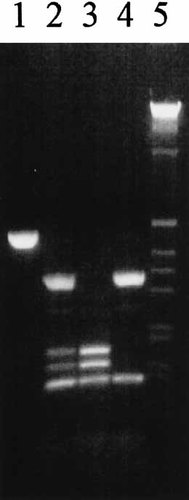
Hha I polymorphism detected by 2.5% agarose gel electrophoresis of restriction digest of PCR-amplified 477 bp fragment. Lane 1, undigested PCR product, 477 bp. Lane 2, heterozygote AG: 338, 181,157, and 130 bp. Lane 3, homozygote GG: 181, 157, and 130 bp. Lane 4, homozygote AA: 338 and 130 bp. Lane 5, 1 kb marker.
Statistical Analysis
Departure from Hardy Weinberg equilibrium (HWE) was assessed using the χ2 goodness of fit test. Statistical significance of differences between distributions for alleles and genotypes was assessed using the Pearson χ2 test.
Allele and genotype distributions are shown in Tables I and II. The genotype distribution was close to that expected under HWE for comparison group 1 (χ2 = 1.10, 1 df, P > 0.1), group 2 (χ2 = 0.05, 1 df, P > 0.1), and for the combined groups (χ2 = 0.62, 1 df, P > 0.1). Genotype distributions in the two comparison groups did not differ significantly (χ2 = 1.19, 2 df, P = 0.55). Therefore, subsequent analyses were performed on the combined comparison groups. There was no significant difference between genotype (χ2 = 1.22, 2 df, P = 0.54) or allele (χ2 = 0.06, 1 df, P = 0.86) distributions in bipolar individuals and the comparison group.
| Allele | A | G | Total |
|---|---|---|---|
| Comparison group | 315 (0.52) | 287 (0.48) | 602 (1.0) |
| Affected group | 331 (0.53) | 293 (0.47) | 624 (1.0) |
- * Frequencies in parentheses. A and G identify the nucleotide base present at position 1832 in the Wolfram gene.
| Genotype | AA | AG | GG | Total |
|---|---|---|---|---|
| Comparison group | 79 (0.26) | 157 (0.52) | 65 (0.22) | 301 (1.0) |
| Affected group | 91 (0.29) | 149 (0.48) | 72 (0.23) | 312 (1.0) |
- * Frequencies in parentheses. A and G identify the nucleotide base present at position 1832 in the Wolfram gene.
When male and female results were analyzed separately, the difference between genotype (χ2 = 6.18, 2 df, P = 0.046) and allele (χ2 = 4.74, 1 df, P = 0.030) distributions for male bipolars and the male comparison group reached conventional levels of statistical significance, the allele not containing the Hha I cutting site being more frequent in affected (0.57) than in unaffected individuals (0.47). However, when corrected for multiple testing, the differences became nonsignificant and were balanced by an opposing trend in females, the allele containing the Hha I site being more frequent in the bipolar group than in the comparison subjects (χ2 = 2.42, 2 df, P = 0.297 for genotype distribution, and χ2 = 2.19, 1 df, P = 0.139 for allele distribution).
RESULTS AND DISCUSSION
In a large case-control study of U.K. Caucasians we have found no evidence for association between bipolar illness and alleles at the A1832G polymorphism within exon 8 of the Wolfram gene. We can, therefore, conclude with a high degree of confidence that variation at this polymorphism does not have a direct pathogenic influence of major or even modest size on susceptibility to bipolar disorder in this population. Using a test size of 0.05 our study has power exceeding 94% to detect an association with an effect size, as measured by an odds ratio (OR) of 1.5, and 83% to detect an OR = 1.4. However, caution is required in interpreting any negative association study result. It remains possible that variation at A1832G could exert a minor (OR < 1.4) pathogenic influence. Further, it is well known that linkage disequilibrium may be absent even over short distances so it remains possible that variation at other polymorphisms at or near the Wolfram gene may influence susceptibility to bipolar disorder. It is of interest that this chromosome region has a high rate of mutation [Alitto et al., 1991; Hubert et al., 1994], a feature that will inevitably reduce the likelihood of identifying linkage disequilibrium.
CONCLUSIONS
Although we have failed to find evidence implicating the Wolfram gene in predisposition to bipolar disorder, a more extensive evaluation of this gene will clearly require mutation/polymorphism detection in a sample of bipolar patients, including affected members of families showing linkage to the region, followed by association studies where appropriate. The search for the susceptibility gene on 4p16 is also likely to require systematic linkage disequilibrium and candidate gene studies across this chromosomal region.




