Ultrasonographic detection of orbicularis oris defects in first degree relatives of isolated cleft lip patients
Abstract
The phenotypic variability of non-syndromic cleft lip (CL) is broad. We demonstrate that the prevalence of orbicularis oris (OO) muscle anomalies, detectable only by ultrasound, is higher in first-degree relatives of individuals with overt CL than in the general population. These findings suggest that occult OO defects may be part of the spectrum of the CL phenotype, that offspring of individuals with such defects are at an increased risk to develop overt CL, and that ultrasound may be a useful tool in future population studies designed to identify CL susceptibility genes. Am. J. Med. Genet. 90:155–161, 2000. © 2000 Wiley-Liss, Inc.
INTRODUCTION
The severity of cleft lip (CL) varies considerably from complete bilateral CL and palate at one end of the spectrum to a minimal CL at the other. The minimal CL is an upper lip defect manifest by minor anomalies such as vermilion notching, philtral flattening, and nostril asymmetry [Lehman and Artz, 1976]. We described a defect of the upper lip even more subtle in presentation than the minimal CL [Martin et al., 1993] and named it the subepithelial CL. We coined the term to describe an aberration of the orbicularis oris muscle and its surrounding connective tissue visible only by histological examination. We postulated that if the CL phenotypic spectrum were broadened to include the subepithelial CL it might have a significant impact on population studies directed towards identifying major genes involved with CL development. Unfortunately, the occult nature of the subepithelial CL precludes any practical determination of its applicability in population studies of clefting unless a noninvasive method of detecting it can be used. The purpose of this study is to present data that suggest ultrasound can be used to detect defects of the orbicularis oris muscle in otherwise clinically normal individuals and that such defects are seen at higher frequency in first degree relatives of patients with overt isolated CLs than in a control population.
MATERIALS AND METHODS
The families of 21 children from the University of California, Irvine craniofacial clinic with non-syndromic isolated CL (with or without clefting of the primary and/or secondary palate) were identified and consented to participate; 52 control subjects were also enrolled. The two criteria for eligibility as a control subject were a negative family history of CL or palate in three generations and absence of any minimal cleft features. All control subjects and each available parent and sib of the CL index case were examined for any minor anomalies of the upper lip/nose/alveolar ridge. Controls and relatives then underwent an ultrasound scan of the upper lip using a 10-MHz high-resolution ATL2000® ultrasound probe. The probe was applied to the philtral area of the upper lip between the nasal columella and the vermilion. After the application of standard ultrasound gel, the upper lip was imaged in the axial plane so that the surface of the philtrum corresponded to the most superior portion of each image and the alveolus (with teeth) corresponded to the most inferior portion of each image. Each scan was recorded on videotape and hardcopy prints of the left and right sides of the lip were made. The only identifying information recorded on the photographs and videotape was a non-sequential randomized number assigned to each study subject. A single ultrasonographer (KM) performed all ultrasound studies, and no conclusions regarding the orbicularis oris were recorded at the time of the study. At the completion of the study period, three reviewers (KM, RM, VH) independently analyzed all photographs and scored each scan either as positive (a defect of orbicularis oris was thought to be present) or negative (the orbicularis oris was thought to be normal). No access to any identifying data linking the randomized scan number to the study subjects was available. Several CL patients allowed us to use ultrasonography to examine their upper lip in an effort to visualize their orbicularis oris anatomy subsequent to repair as did a child with an unrepaired minimal CL.
Segregation analysis on the study sample was performed as follows. The orbicularis oris for each study subject was defined as abnormal if any two of the three reviewers scored a scan as positive. The family data with respect to scan scores were tabulated within mating type by sibship size. However, we were unable to subject these tables to formal segregation analysis because of the small sample sizes so we were restricted to simple observation of the segregation patterns.
RESULTS
The frequencies of positive scores compared with negative scores by each reviewer are set forth in Table I. The ultrasound scores by two reviewers (KM, RM) demonstrate a significant increase of positive scores in first-degree relatives versus controls (49 and 44% versus 19%, respectively). The increase of positive scores in first-degree relatives noted by VH (18% versus 10%) is less dramatic and not statistically significant. If the subjects on whom a score was agreed upon by at least 2 of the 3 reviewers are considered together, a statistically significant increase (P < 0.002) between the presence of a positive score in first-degree relatives versus controls (40% versus 13%, respectively) is noted.
| Parameters | Reviewers | Combineda | ||
|---|---|---|---|---|
| KM | RM | VH | ||
| First degree relative | ||||
| Orbicularis oris defect | 27 (49%) | 24 (44%) | 10 (18%) | 22 (40%) |
| Orbicularis oris normal | 28 (51%) | 31 (56%) | 45 (82%) | 33 (60%) |
| Control | ||||
| Orbicularis oris defect | 10 (19%) | 10 (19%) | 5 (10%) | 7 (11%) |
| Orbicularis oris normal | 42 (81%) | 42 (81%) | 47 (90%) | 45 (89%) |
| Level of significance | P = 0.001 | P = 0.007 | P = 0.20 | P = 0.002 |
| Chi-square | χ2 = 10.54 | χ2 = 7.34 | χ2 = 1.63 | χ2 = 9.53 |
- a OO defect is categorized as present here if at least two of the three reviewers gave a positive score.
Table II summarizes the tabulation of families by diagnosis and mating type. Of note is that all three sibs for the two ‘positive × positive’ matings are also positive and more sibs are positive in the ‘positive × negative’ matings than in ‘negative × negative’ matings (3 versus 1, respectively).
| Mating type | Total number of sibs (including index case) | Number of positive sibs (including index case) | Number of families with this structure |
|---|---|---|---|
| Positive × positive | 1 | 1 | 1 |
| 4 | 4 | 1 | |
| Total families | 2 | ||
| Positive × negative | 1 | 1 | 3 |
| 2 | 1 | 2 | |
| 2 | 2 | 3 | |
| 3 | 1 | 2 | |
| Total families | 10 | ||
| Negative × negative | 1 | 1 | 1 |
| 2 | 1 | 1 | |
| 2 | 2 | 1 | |
| 4 | 1 | 1 | |
| Total families | 4 | ||
| Positive × unknown | 1 | 1 | 1 |
| Total families | 1 | ||
| Negative × unknown | 1 | 1 | 3 |
| 2 | 1 | 1 | |
| Total families | 4 |
- * Total number of families was 21.
Figure 1A–C are representative of scans for which all three reviewers scored the anatomy as negative (normal). Note the distinctive uninterrupted hypoechoic (dark) appearance of the orbicularis oris muscle from one side to the other. Figure 2A–C are examples of scans for which all three reviewers scored the anatomy as positive (abnormal). Note the interruption of the hypoechoic orbicularis oris by a focal echogenic area as compared with Figure 1. Figure 3 is taken from a child with a repaired cleft, a procedure that includes a surgical union of the orbicularis oris muscle. Note the echogenic area within the orbicularis oris similar to that demonstrated in Figure 2. This echogenic focus is at the site of the surgical union. The photograph in Figure 4 is illustrative of scans scored positive by two reviewers (KM, RM) and negative by one reviewer (VH). The young boy in Figure 5 presented for surgical evaluation because of his minimal cleft represented by unilateral vermilion notching, philtral flattening, and ala nasi deformity. Figure 6A and B show the lip ultrasound scan from this child prior to repair and that of his clinically normal father. The child's orbicularis oris underlying the area of his unrepaired minimal cleft is similar in appearance to that in Figure 2. Note the resemblance of the father's orbicularis oris to these images as well.
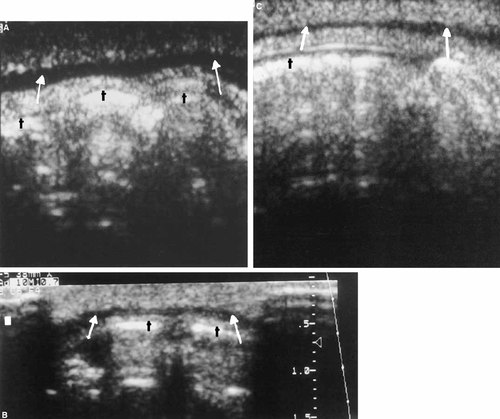
Three (A–C) representative examples of ultrasound images taken in the axial plane regarded by all reviewers as normal. White arrow points to the hypoechoic orbicularis oris muscle. The bright objects posterior to the orbicularis oris are teeth (t).
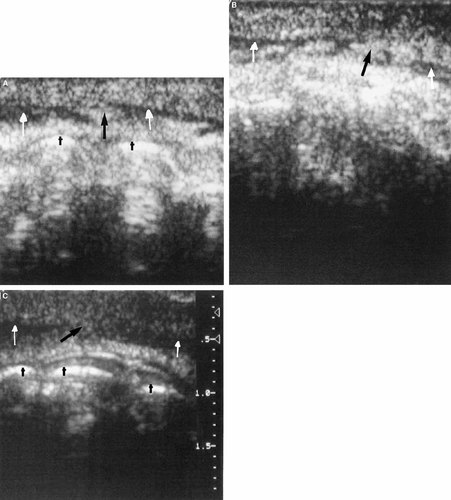
Three (A–C) representative examples of images regarded by all reviewers as abnormal. Black arrow points to an echogenic area that interrupts the normal hypoechoic orbicularis muscle as demonstrated in Figure 1.

Ultrasound image of the orbicularis oris at the sight of a unilateral cleft lip repair. Note the similar appearance to those images in Figure 2.
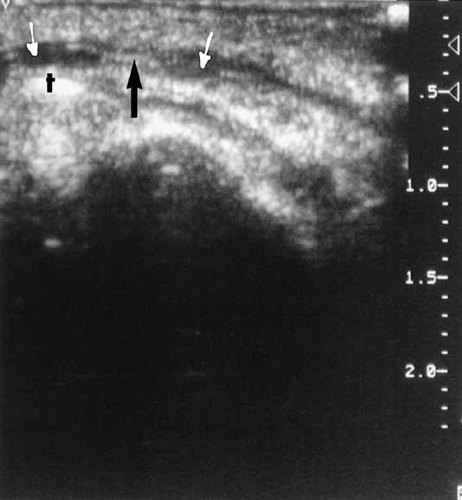
Example of a scan scored positive by two reviewers and negative by one reviewer.

Child with a minimal cleft lip prior to repair. Note the left-sided philtral flattening and nostril asymmetry in addition to the slight vermilion scar.
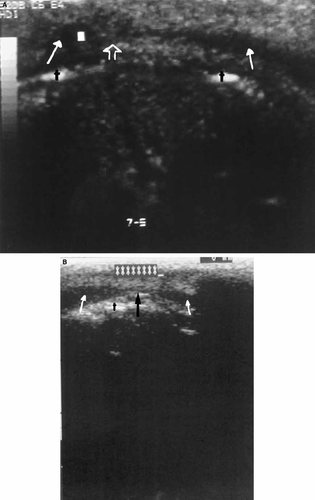
A: The lip ultrasound image of the child in Figure 5 prior to surgery taken at the site of the left philtral flattening superior to the small vermilion scar. Note the interrupted (echogenic) appearance of the orbicularis oris (open arrow). B: Lip ultrasound of the child's clinically unaffected father that also demonstrates an echogenic interruption (black arrow) of the orbicularis oris.
DISCUSSION
Several investigators [Lindral et al., 1997; Marazita et al., 1992; Mitchell, 1997; Romitti et al., 1999] have postulated that the occurrence of isolated CL is influenced to a large extent by several susceptibility gene loci. Much effort is now being put forth to identify these genes using family and population studies, however one limitation of such studies is the definition of ‘affected’ and ‘unaffected’ study subjects. Bixler [1991] and Melnick [1992] both recognized that under-identification of ‘affected’ relatives might be a major problem because such studies are not designed to specifically identify relatives who are only mildly affected. Such under-identification would be even more significant if the upper lip defects in affected relatives are visually occult. The increase of apparent orbicularis oris anomalies in first-degree relatives of overt CL patients compared with a control population in this study is evidence that such an occult defect of the upper lip does exist. It is not surprising that a minor anomaly of the mesodermally derived orbicularis oris muscle should be part of the spectrum of CL since overt CL formation is the result of defects in the development of the mesodermally derived maxillary processes.
If a large-scale study confirms that this occult orbicularis oris defect occurs at a higher incidence in first-degree relatives of CL patients, then its identification within large pedigrees could be a useful adjunct in population studies designed to localize genes involved in CL susceptibility. Such genes have been referred to as “morphogenes” in reports of parental cephalometric craniofacial studies that also demonstrate distinct differences in craniofacial morphology between parents who have offspring with CL and controls [Mossey et al., 1998].
The simplest hypothesis for inheritance of the observed orbicularis oris defect is that it is due to an allele at a single locus. Our sample of family data is too small to adequately test this hypothesis, but two observations can be made. First, all sibs analyzed in our two ‘positive × positive’ matings were also positive. Second, more positive children were found with just one parent negative than with both parents negative. These findings are most consistent with recessive inheritance as a single allele model. The observation of a positive child whose parents are both negative also supports this hypothesis, as the mating could be a heterozygous cross, especially given the 13% prevalence of the ‘positive&’ trait in our control group. Using this prevalence in the general population we estimate that approximately 50 offspring from ‘positive × negative’ matings would be necessary to test for recessive inheritance. We would choose to study the ‘positive × negative’ mating type because these data are most informative. The ‘positive × positive’ matings would also be quite useful but their frequency would be limited if our 13% prevalence figure for the ‘positive’ trait is accurate. If such a high prevalence of this trait in a control population is confirmed in a larger study, the intriguing challenge shall be to find the triggers associated with development of overt CL.
This study clearly has limitations. First and foremost is the assumption that the echogenic areas on ultrasound that form the basis for a ‘positive’ score actually represent a physical defect of the orbicularis oris. The subepithelial CL as originally reported [Martin et al., 1993] was manifest by an interruption or gap in orbicularis oris development. Our hypothesis is that such a defect should result in some structural morphological change that persists after birth and is the basis for the ultrasonic ‘defect’ we observed. The litmus test of this hypothesis would be to demonstrate histological orbicularis oris defects in subjects scored as ‘positive,’ a correlation we are unlikely to ever have the opportunity to make for obvious reasons. However, the remarkable similarity of the sonographic ‘defect’ in clinically normal individuals to those children with CLs who have had a surgical union of their orbicularis oris (compare Fig. 2 with Fig. 3) and to the child with an unrepaired minimal cleft (Fig. 6A) suggests that such a persistent morphological change does occur. Perhaps there is an embryological attempt at repair of the underlying orbicularis oris defect with a deposition of collagen at the edges of the orbicularis, as in a surgical repair (scar), that represents the ultrasonic defect detected in this study.
Another problematic aspect of this study is that we used a discontinuous method of scoring orbicularis oris ultrasound exams (i.e., either positive or negative). A quantitative approach to assessment of normal and abnormal orbicularis oris development might be more appropriate given the likely construct that a continuum of differences exists in development of specific craniofacial structures [Johnston and Hunter, 1989; Johnston and Bronsky, 1995]. This point is illustrated by Figure 7, which shows an orbicularis oris muscle without a typical echogenic interruption as seen in many of the ‘positive’ cases but nonetheless with an abnormally thin appearance compared with the ‘normal’ images in Figure 1. A quantitative approach such as volumetric measurement based on standards for age and sex should allow for a more refined interpretation of normal and abnormal. A quantitative approach would also give a direct measure of reproducibility and inter-reviewer reliability, and would allow for replication in independent studies. Inter-reviewer reliability was problematic in this study in that the actual number of scans scored positive by VH in both the study and control groups was notably less than that of the other two reviewers. However, an important observation is that the other two reviewers both agreed on all 15 of VH's positive scans. Such agreement implies that a discernible difference between ‘positive’ and ‘negative’ does exist. The photograph in Figure 4 suggests that VH was subjectively conservative in scoring scans as positive. Larger studies will be necessary to better assess the degree of inter-reviewer reliability. Such reliability needs to be established if this procedure is going to be of practical use in identifying individuals with orbicularis oris aberrations in future population studies of CL.
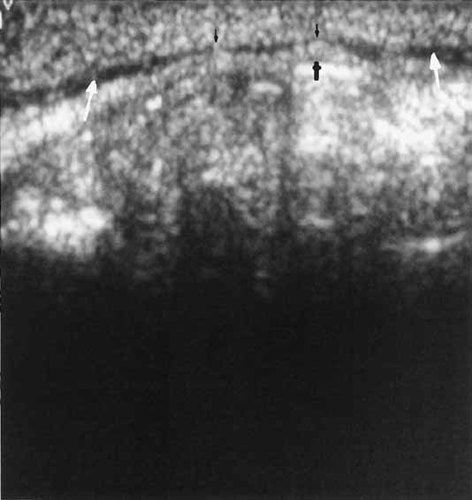
An example of orbicularis narrowing (small black arrows) but without overt, echogenic interruption.
In summary, we have shown in this study that ultrasonography may be a practical tool to further refine the phenotype of abnormal embryological maxillary process development as it relates to the genesis of CL. Defects of the orbicularis oris muscle may be a risk factor for having offspring with overt CL and genes involved with the development of this muscle may be likely candidates for clefting susceptibility.




