Chudley-Mccullough syndrome: Bilateral sensorineural deafness, hydrocephalus, and other structural brain abnormalities
Abstract
The Chudley-McCullough syndrome, an autosomal recessive condition first reported by Chudley et al. [1997], comprises profound sensorineural hearing loss and hydrocephalus secondary to an obstruction of the foramen of Munro. We describe two more sibs with this condition. One girl had sensorineural hearing loss and hydrocephalus due to obstruction of the foramen of Munro. Incidentally she was also found to carry a full mutation in the FMR1 gene. The older sister had profound sensorineural hearing loss and hydrocephalus not due to obstruction of the foramen of Munro; she also had callosal dysgenesis, gray matter heterotopia, cortical dysplasia, and cerebellar dysgenesis. Thus, the Chudley-McCullough syndrome may include hydrocephalus not necessarily related to obstruction of the foramen of Munro and other structural brain abnormalities. Am. J. Med. Genet. 90:127–130, 2000. © 2000 Wiley-Liss, Inc.
INTRODUCTION
Recently Chudley et al. [1997] described a Canadian family of Mennonite origin in which a brother and sister had bilateral sensorineural deafness and hydrocephalus due to an obstruction of the foramen of Munro. Autosomal recessive inheritance was postulated based on parental consanguinity, the absence of congenital infections, and the fact that sibs of both sexes were affected.
Here we describe two sisters of Mennonite descent with hydrocephalus and profound bilateral sensorineural deafness. One sister has hydrocephalus not due to obstruction of the foramen of Munro and structural brain abnormalities not previously described in association with this condition; we suggest the eponym Chudley-McCullough syndrome.
CLINICAL REPORT
Case K.K.
K.K., age 6 years, is the youngest of four children born to a healthy 31-year-old mother and a 30-year-old father. The pregnancy with K.K. was uncomplicated, and there was no history of exposure to any known teratogens. Hydrocephalus was diagnosed by antenatal ultrasound one day prior to delivery. She was born at 41 weeks of gestation by scheduled Cesarean section, weighing 4,390 g (> 95th centile) with a head circumference of 43.5 cm (>> 95th centile) and a length of 56 cm (> 95th centile). Apgar scores were 7 and 8 at 1 and 5 min, respectively. Ultrasound and computed tomography (CT) of the head showed enlargement of the lateral ventricles thought to be compatible with aqueductal stenosis. Results of sepsis work-up and TORCH screen were negative. A ventriculo-peritoneal shunt was inserted at age 2 days due to increasing intracranial pressure. Chromosomes were normal (46,XX), however a fragile site at Xq27.3 was detected in 20% of metaphases. Shunt revision was performed at age 10 weeks because of obstruction. At age 5 years, after a seizure, a left ventriculo-peritoneal shunt was inserted following evidence of increased pressure on the left side of the brain due to a loculated ventricle.
K.K. has always demonstrated significant developmental delay. An audiological assessment at age 15 months confirmed severe bilateral sensorineural hearing loss. She was treated with Risperidone® for a short attention span and behavior problems. DNA testing for fragile X syndrome was requested at age 6 years because of the clinical and family history. She was found to carry a full mutation expansion in the FMR1 gene. Given the clinical similarities (hydrocephalus and deafness) and the identical ethnic background to the family described by Chudley et al. [1997], a re-evaluation of the CT scans was requested and found to show obstruction of the foramen of Munro and not aqueductal stenosis as reported previously (Fig. 1).
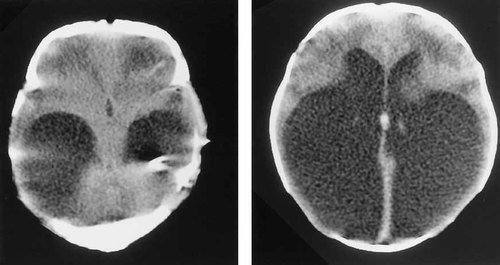
Axial CT brain scan of K.K. Note enlarged temporal horns and normal third ventricle (left). Enlarged occipital horns of lateral ventricles (right).
Case R.K.
R.K., a 15-year-old girl, is the eldest sib of K.K. The pregnancy with R.K. was uncomplicated without exposure to any known teratogens. Delivery was at term by Cesarean section because of cephalo-pelvic disproportion. Apgar scores were 8 and 8 at 1 and 5 min, respectively. Birth parameters were all at or above the 90th centile. The parents were first concerned about her hearing at age 5 to 6 months. Audiological assessment at age 11 months confirmed severe to profound sensorineural hearing loss. A developmental assessment at age 25 months found her to be functioning within normal limits except in areas directly affected by her hearing impairment. At age 15 years, she was in a modified grade 9 classroom with access to a teacher's aide. Chromosomes were normal (46,XX). Fragile-X testing showed her to have 32 and 32 CGG repeats. An elective CT scan of the head was requested because of her sister (K.K.) and the similarities to the cases described in the report by Chudley et al. [1997]. Prior to her scheduled appointment, R.K. suffered a severe trauma to the head with loss of consciousness and required neuroimaging. CT scan of the head showed a dilated right lateral ventricle and probable callosal dysgenesis. There was no definite evidence of a foramen of Munro obstruction. A hyperdense focus in the left occipital horn was present, suggestive of a mild left intraventricular bleed. Given these unusual findings, magnetic resonance imaging of the brain was requested. It confirmed the findings on CT scan including dysgenesis of the corpus callosum but also found evidence of bifrontal cortical dysplasia, gray matter heterotopia, and abnormal gray and white matter architecture of the cerebellar hemispheres (Fig. 2). She is being followed closely for potential ventriculo-peritoneal shunting.
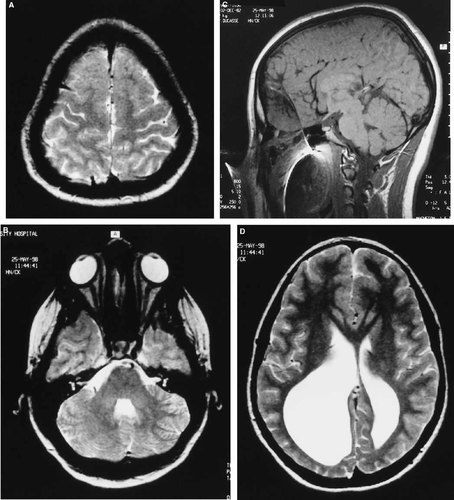
Magnetic resonance imaging of R.K.'s brain. A: Axial T2 image showing frontal lobe cortical dysplasia (polymicrogyria). B: Axial T2 image showing cerebellar dysgenesis. C: Sagittal T1 image showing callosal dysgenesis (partial agenesis) and polymicrogyria. D: Axial T2 image showing enlarged occipital horns of lateral ventricles (R>) and bifrontal gray matter heterotopia. Note image distortion, especially of the sagittal view, is related to metal artifact from the patient's braces.
Family History
The children (K.K. and R.K.) were born to nonconsanguineous parents of Mennonite descent. There is no other history of hydrocephalus or sensorineural hearing loss. This is a known fragile-X-syndrome family.
DISCUSSION
Chudley et al. [1997] previously described two children of Mennonite descent with an autosomal recessive condition characterized by sensorineural deafness and hydrocephalus due to obstruction of the foramen of Munro. In this report we describe two sisters, also of Mennonite origin, both with hydrocephalus and profound sensorineural hearing loss. The younger child has hydrocephalus secondary to obstruction of the foramen of Munro. The older sister has hydrocephalus with a dilated right ventricle with no evidence of obstruction of the foramen of Munro. In addition, this individual had evidence of callosal dysgenesis, cortical dysplasia, gray-matter heterotopia, and cerebellar dysgenesis.
The younger sister almost certainly has the same disorder described in the report by Chudley et al. [1997]. Because these two sisters both have profound sensorineural hearing loss and hydrocephalus, it is very likely that they have the same genetic condition. The clinical differences observed most likely represents the variability of this condition. If this reasoning is accepted, then the list of structural brain abnormalities should include those identified in the older sister. Thus, hydrocephalus in the Chudley-McCullough syndrome is not necessarily due to obstruction of the foramen of Munro and may be associated with other structural brain abnormalities such as cortical dysplasia, callosal dysgenesis, gray-matter heterotopia, and cerebellar dysgenesis.
It is unlikely that the structural brain abnormalities described in this report are related to the fragile X syndrome. Structural brain abnormalities reported in the fragile X syndrome have been nonspecific or have involved the cerebellar vermis [Reiss et al., 1991]. The child diagnosed with fragile X syndrome has similar clinical features and identical neuroradiological findings to the non-fragile X children in the report by Chudley et al. [1997]. The previously unreported structural brain abnormalities associated with this condition were found in the adolescent sister who does not have fragile X syndrome. Neuroimaging of other relatives was not undertaken because there were no clinical indications.
Although these children were born to nonconsanguineous parents, autosomal recessive inheritance of a single gene is the most likely mode of inheritance given that the parents in the report by Chudley et al. [1997] were consanguineous. It is unlikely that two or more separate autosomal recessive mutations are responsible for the sensorineural hearing loss and structural brain abnormalities. X-linked recessive inheritance is less likely given that three females and only one male have so far been identified with this condition. Gonadal mosaicism for an autosomal dominant mutation and unbalanced segregation of a cryptic chromosome translocation cannot be ruled out.
Finally, it would be interesting to see if other individuals with the Chudley-McCullough syndrome are described to determine the full extent of associated structural brain abnormalities. Given that two more cases were identified shortly after the initial reporting of this condition, it is possible that this disorder is more common amongst the Mennonite population than previously thought. Possibly non-Mennonite individuals with profound sensorineural hearing loss may have similar neuroradiological findings. We propose that neuroimaging of the brain be considered in all individuals with profound sensorineural hearing loss, especially those of Mennonite background.
Acknowledgements
We thank Drs. Karen Tong and Rodney Friedman for their assistance in the interpretation of the imaging studies. We also thank Katherine Tunnicliffe for her expert secretarial assistance.




